Preparation method of pyridine derivative
A technology of pyridine and hydroxypyridine, which is applied in the field of new preparation of pharmaceutical intermediates, and can solve problems such as multi-drug resistance, abuse, and irrational application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
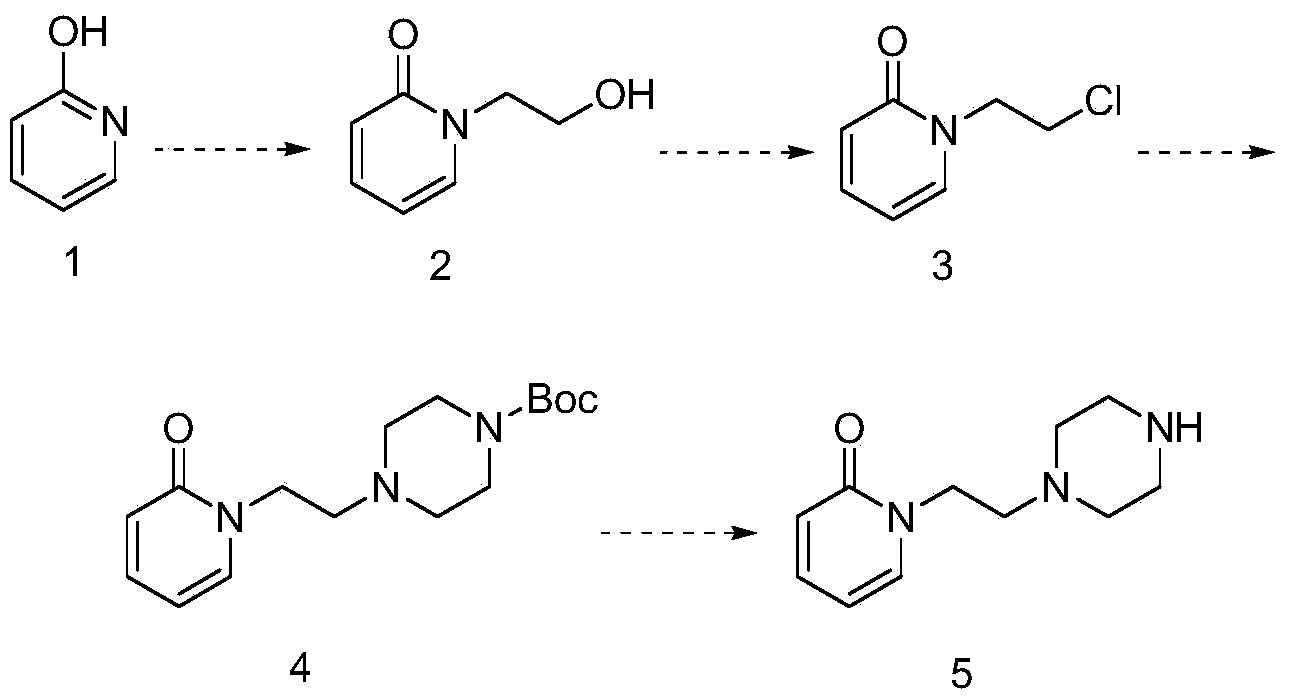
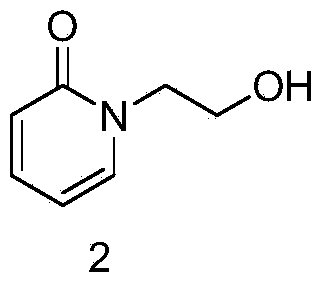
[0024] (1) Synthesis of 1-(2-hydroxyethyl)pyridin-2(1H)-one
[0025] Add 20g of 2-hydroxypyridine to 500ml of N,N-dimethylformamide, add 9g of anhydrous potassium carbonate, then add 40g of 2-bromoethanol, heat and reflux and stir for 8 hours, cool to room temperature, add water for extraction, and separate the liquids , dried, concentrated, and the residue was subjected to column separation to obtain 23 g of 1-(2-hydroxyethyl)pyridin-2(1H)-one.
[0026] (2) Synthesis of 1-(2-chloroethyl)pyridin-2(1H)-one
[0027] Add 22g of 1-(2-hydroxyethyl)pyridin-2(1H)-one to 140ml of thionyl chloride, heat to reflux for 6 hours, cool to room temperature, remove excess thionyl chloride under reduced pressure, then add water and Ethyl acetate, extraction and separation, the organic phase was collected, liquid separation, drying, and concentration, and the residue was separated on a silica gel column to obtain 18 g of 1-(2-chloroethyl)pyridin-2(1H)-one.
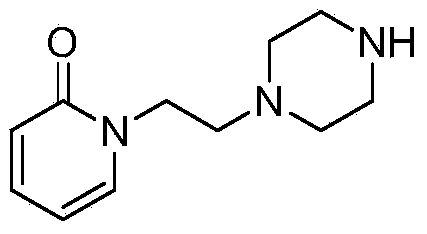
[0028] (3) Synthesis of tert-butyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com