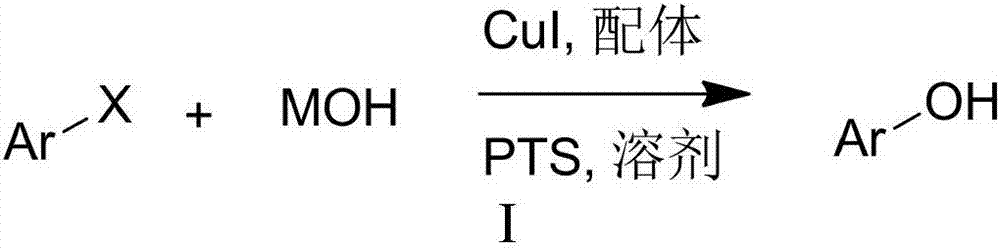
Hydroxylation method of halogenated aromatic compound
An aromatic compound and hydroxylation technology, which is applied in the preparation of organic compounds, the preparation of carbon-based compounds, chemical instruments and methods, etc., can solve the problems of difficult reaction of substrates and expensive ligands, and achieves low price and good application prospects. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of 4-methoxyphenol
[0036]
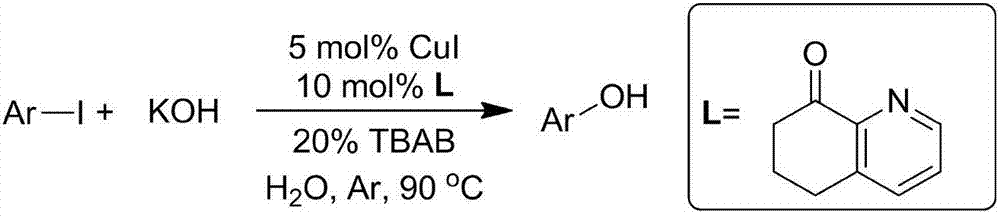
[0037] In a reaction tube sealed at one end, 234mg p-methoxy iodobenzene (MW=234, 1.0 mmol) was added, and then 280mg KOH (MW=56,5mmol), 14.7mg 6,7-dihydroquinoline-8 were added sequentially (5H)-ketone (MW=147, 0.1mmol), 9.5mg CuI (MW=190, 0.05mmol), 64mg tetrabutylammonium bromide (TBAB) (MW=320, 0.2mmol) and 1mL water, in argon Under the protection of gas or nitrogen, the reaction was stirred at 90°C for 24 hours. After the reaction liquid was cooled, 5 ml of 30% hydrochloric acid was added, and then the reaction mixture was extracted three times with 30 ml of ethyl acetate. The extracts were combined, dried, and distilled under reduced pressure. It was separated through a silica gel column (the eluent was petroleum ether: ethyl acetate = 3:1) to obtain 115 mg of 4-methoxyphenol product, with a yield of 93%.
[0038]
[0039] 1 H NMR(CDCl 3 )δ6.79(m,4H),5.88(br,1H),3.77(s,3H); 13 C NMR(CDCl 3 ) δ153.5, 149.5, 116.1, 114...
Embodiment 2
[0040] Example 2 Preparation of 4-methoxyphenol
[0041]
[0042] According to the method described in Example 1, the difference is that the base used is NaOH, and 200 mg of NaOH and 4-methoxyiodobenzene (234 mg, 1.0 mmol) are stirred for reaction for 24 hours. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 3:1), yield: 81%;
[0043] 1 H NMR(CDCl 3 )δ6.79(m,4H),5.88(br,1H),3.77(s,3H); 13 C NMR(CDCl 3 ) δ153.5, 149.5, 116.1, 114.9, 55.9.
Embodiment 3
[0044] Example 3 Preparation of 4-methoxyphenol
[0045]
[0046] According to the method described in Example 1, the difference is that the base used is CsOH, and 600 mg of CsOH and 4-methoxyiodobenzene (234 mg, 1.0 mmol) are used to stir and react for 24 hours. The crude product was purified by column chromatography (petroleum ether: ethyl acetate = 3:1), yield: 96%;
[0047] 1 H NMR(CDCl 3 )δ6.79(m,4H),5.88(br,1H),3.77(s,3H); 13 C NMR(CDCl 3 ) δ153.5, 149.5, 116.1, 114.9, 55.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com