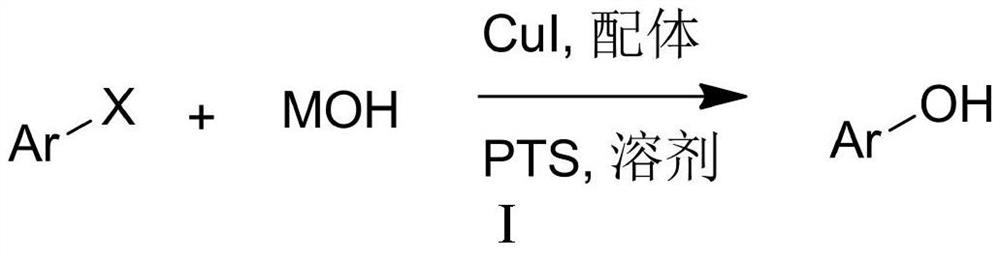
Hydroxylation of Halogenated Aromatic Compounds
A technology of aromatic compounds and hydroxylation, which is applied in the preparation of organic compounds, carbon-based compounds, chemical instruments and methods, etc., can solve the problems of expensive ligands and difficult reactions of substrates, and achieve good application prospects and low prices , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 4-methoxyphenol
[0036]
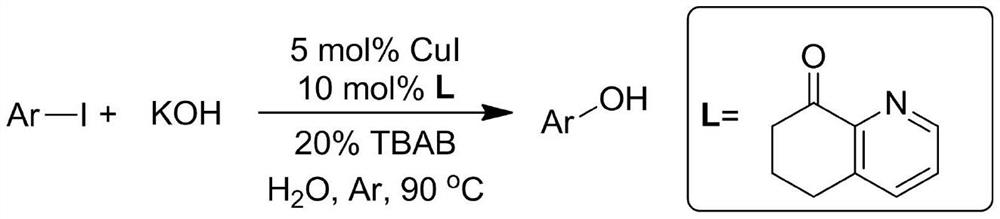
[0037] In a sealed reaction tube at one end, add 234mg p-methoxyiodobenzene (MW=234,1.0 mmol), then add 280mg KOH (MW=56,5mmol), 14.7mg 6,7-dihydroquinoline-8 (5H)-ketone (MW = 147, 0.1mmol), 9.5mg CuI (MW = 190, 0.05mmol), 64mg tetrabutylammonium bromide (TBAB) (MW = 320, 0.2mmol) and 1mL water, in argon Under the protection of air or nitrogen, stir the reaction at 90°C for 24 hours. After the reaction liquid is cooled, add 5 ml of 30% hydrochloric acid, then extract the reaction mixture three times with 30 mL of ethyl acetate, combine the extracts, dry, and distill under reduced pressure. Separation on a silica gel column (eluent: petroleum ether: ethyl acetate = 3:1) yielded 115 mg of the product 4-methoxyphenol with a yield of 93%.
[0038]
[0039] 1 H NMR (CDCl 3 )δ6.79(m,4H),5.88(br,1H),3.77(s,3H); 13 C NMR (CDCl 3 ) δ153.5,149.5,116.1,114.9,55.9.
Embodiment 2
[0040] The preparation of embodiment 2 4-methoxyphenol
[0041]
[0042] According to the method described in Example 1, the difference is that the base used is NaOH, NaOH 200mg and 4-methoxyiodobenzene (234mg, 1.0mmol) were stirred and reacted for 24h. The crude product was purified by column chromatography (petroleum ether:ethyl acetate=3:1), yield: 81%;
[0043] 1 H NMR (CDCl 3 )δ6.79(m,4H),5.88(br,1H),3.77(s,3H); 13 C NMR (CDCl 3 ) δ153.5,149.5,116.1,114.9,55.9.
Embodiment 3
[0044] The preparation of embodiment 3 4-methoxyphenol
[0045]
[0046]According to the method described in Example 1, the difference is that the base used is CsOH, and 600 mg of CsOH and 4-methoxyiodobenzene (234 mg, 1.0 mmol) were stirred and reacted for 24 h. The crude product was purified by column chromatography (petroleum ether:ethyl acetate=3:1), yield: 96%;
[0047] 1 H NMR (CDCl 3 )δ6.79(m,4H),5.88(br,1H),3.77(s,3H); 13 C NMR (CDCl 3 ) δ153.5,149.5,116.1,114.9,55.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com