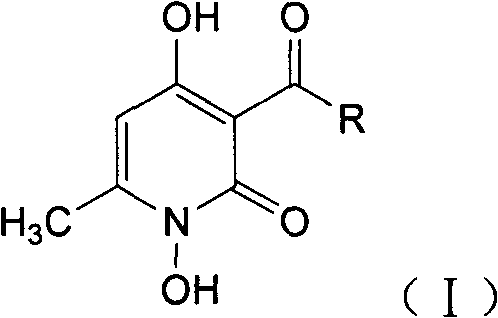
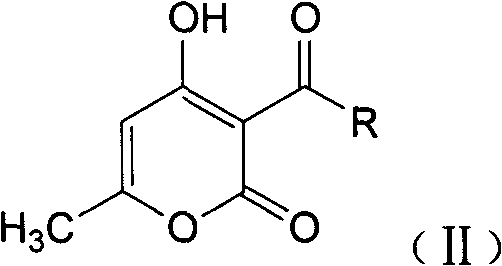
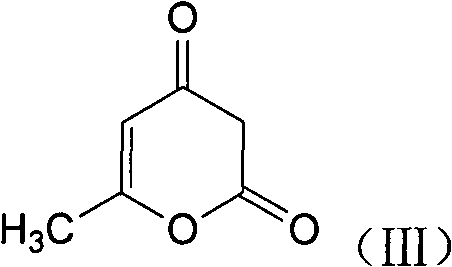
1,4-dyhydroxy-6-methyl-2-pyridine ketone compound, and preparation method and use thereof
A compound, dihydroxy technology, applied in the field of 1,4-dihydroxy-6-methyl-2-pyridone compounds and their preparation and application, can solve side effects, poor metabolic stability, phototoxicity, and cytotoxicity Satisfactory and other issues, to achieve low cytotoxicity, broad-spectrum antibacterial effect, the effect of preventing or treating AIDS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0037] Embodiment 1.1, the preparation of 4-dihydroxy-6-methyl-3-acetyl-2-pyridone
[0038]
[0039] 16.8g (0.1mol) of 1,4-dihydroxy-6-methyl-3-acetyl-2-pyrone was added to a three-neck circle containing 200ml of tetrahydrofuran solution equipped with a KPG stirrer and a thermometer. In the bottom flask, the mixture was warmed to 50 °C. 11.4 g (0.1 mol) of sodium carbonate and 15 g (0.22 mol) of hydroxylamine hydrochloride are added in 5 portions at this temperature within 15 minutes. After stirring at 50° C. for 10 hours, 100 ml of distilled water was added, and tetrahydrofuran was removed under reduced pressure. The residue was added to 3L of ethanol, adjusted to pH 3 with 37% hydrochloric acid, the mixture was cooled to room temperature, and the solid was filtered off. Wash with 100ml methanol and 100ml potable water. The wet paste was stirred with 200 ml of drinking water each at 60° C., filtered with suction and washed with 100 ml of water each to remove residual sa...
Embodiment 21
[0042] Example 2.1, Preparation of 4-dihydroxy-6-methyl-3-acetyl-2-pyridone
[0043] 16.8g (0.1mol) of 4-hydroxyl-6-methyl-3-acetyl-2-pyrone was added to a three-necked round-bottomed flask containing 200ml of aqueous solution with a KPG stirrer and a thermometer, The mixture was warmed to 90°C. 11.4 g (0.1 mol) of sodium carbonate and 15 g (0.22 mol) of hydroxylamine hydrochloride are added in 5 portions at this temperature within 10 minutes. After stirring at 90° C. for 10 hours, it was added to 3 L of methanol, adjusted to pH 3 with 37% hydrochloric acid, the mixture was cooled to room temperature, and the solid was filtered out. Wash with 100ml methanol and 100ml potable water. The wet paste was stirred with 200ml of drinking water at 60°C each time, filtered with suction and washed with 100ml of water each time to remove residual salt, and dried to obtain 10.7g of product with a yield of 58.2% and a melting point of 172-175°C.
[0044] 1 H NMR (DMSO-d 6 , 300MHz) δ: ...
Embodiment 31
[0046] Example 3.1, Preparation of 4-dihydroxy-6-methyl-3-acetyl-2-pyridone
[0047] 16.8g (0.1mol) of 4-hydroxyl-6-methyl-3-acetyl-2-pyrone was added to a three-necked round-bottomed flask containing 200ml of aqueous solution with a KPG stirrer and a thermometer, The mixture was warmed to 90°C. 21.2 g (0.2 mol) of sodium carbonate and 30 g (0.44 mol) of hydroxylamine hydrochloride are added in 5 portions at this temperature within 10 minutes. After stirring at 90°C for 10 hours, cool to room temperature and add 100ml of ethyl acetate for extraction. After proper distillation of ethyl acetate at 50°C, add 8.5ml (0.14mol) of ethanolamine to the ethyl acetate solution, cool and place Crystallization, suction filtration, washing with a small amount of cold ethyl acetate, and vacuum drying gave 16.5 g of 1,4-dihydroxy-6-methyl-3-acetyl-2-pyridone (ethanolamine salt), with a yield of 67.5%. The melting point is 157-160°C.
[0048] 1 H NMR (DMSO-d 6 , 300MHz) δ: 1.82(s, 3H), 2....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com