Synthesis method using ionic liquids
A technology for ionic liquids and uses, applied in the field of synthesis using ionic liquids, can solve the problems of toxicity of diphenyl sulfone, environmental problems and the like, and achieve the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
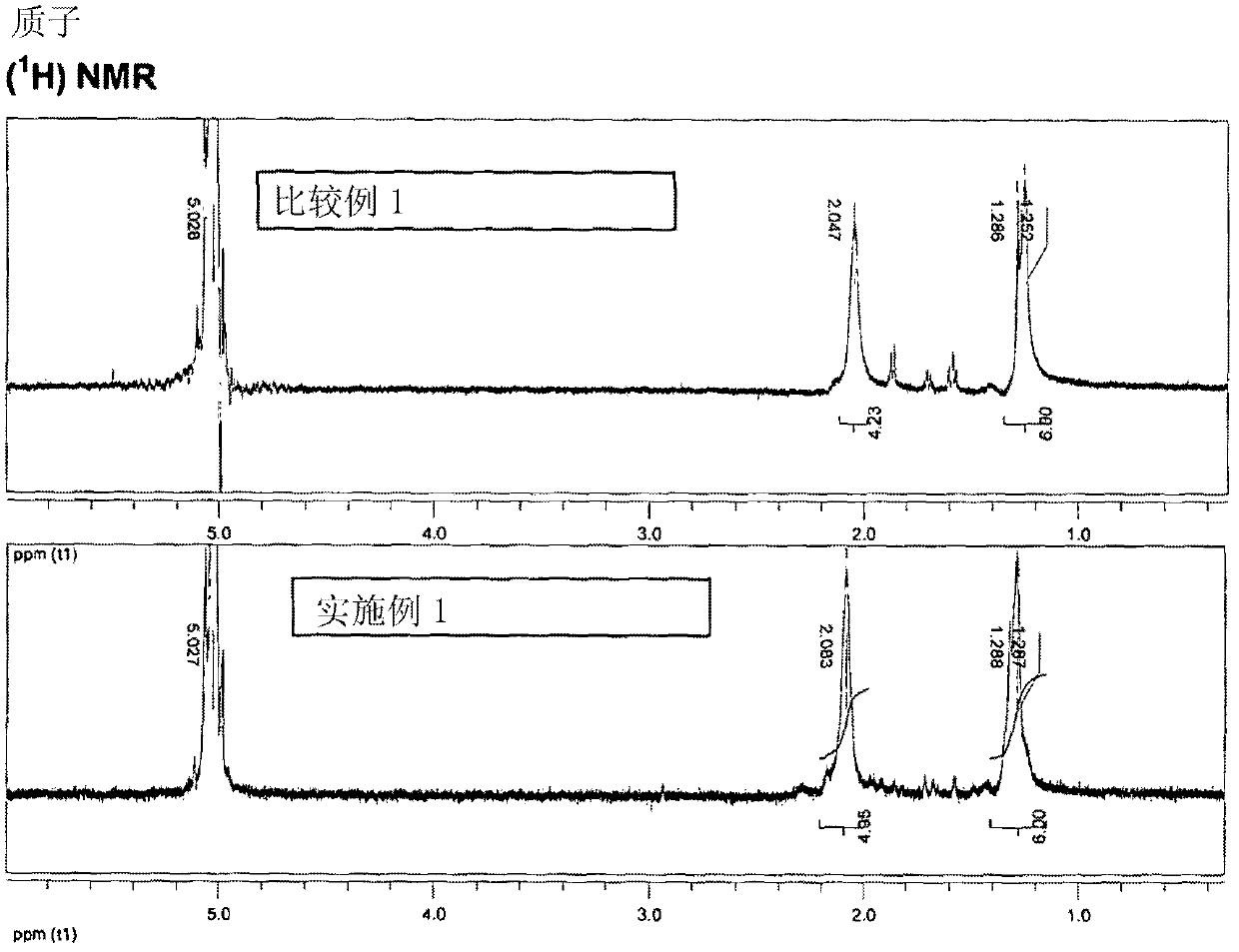
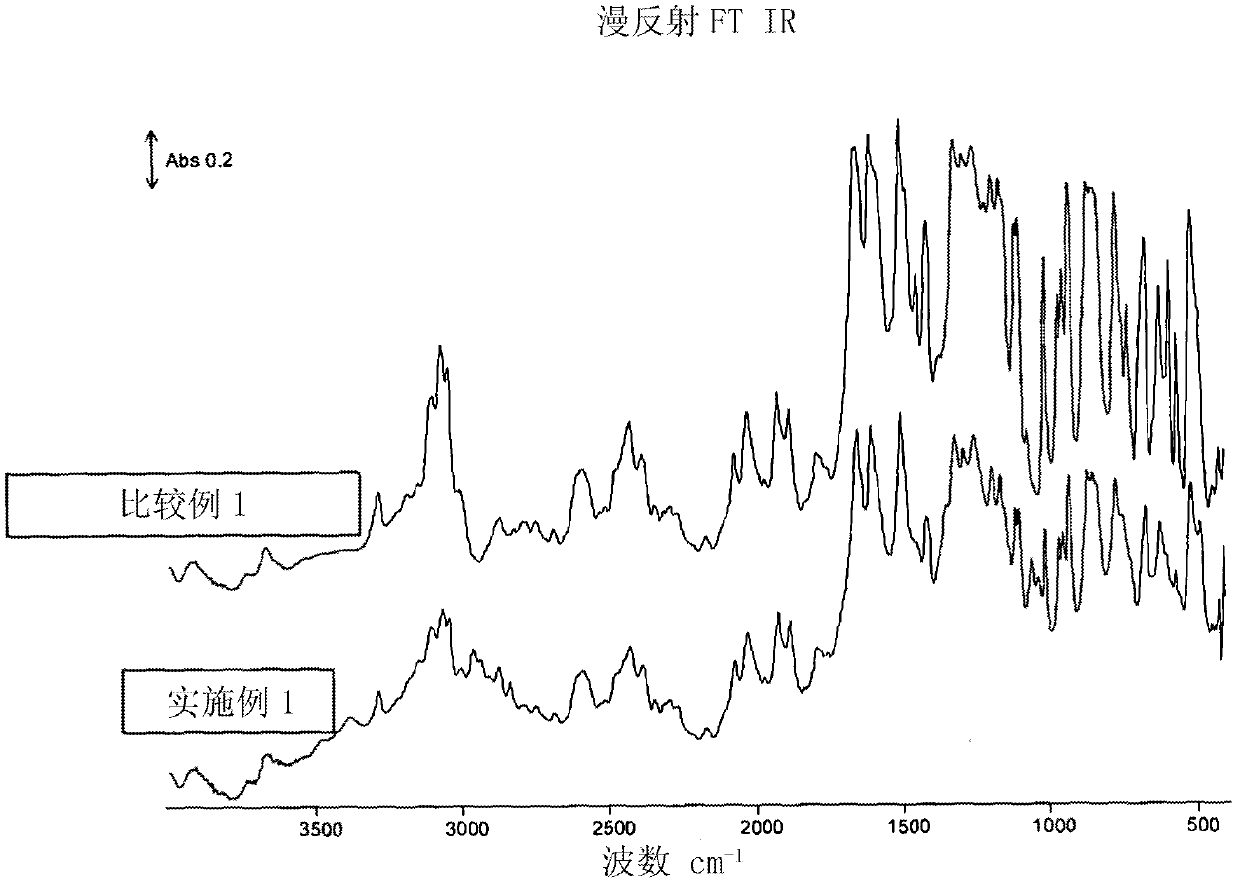
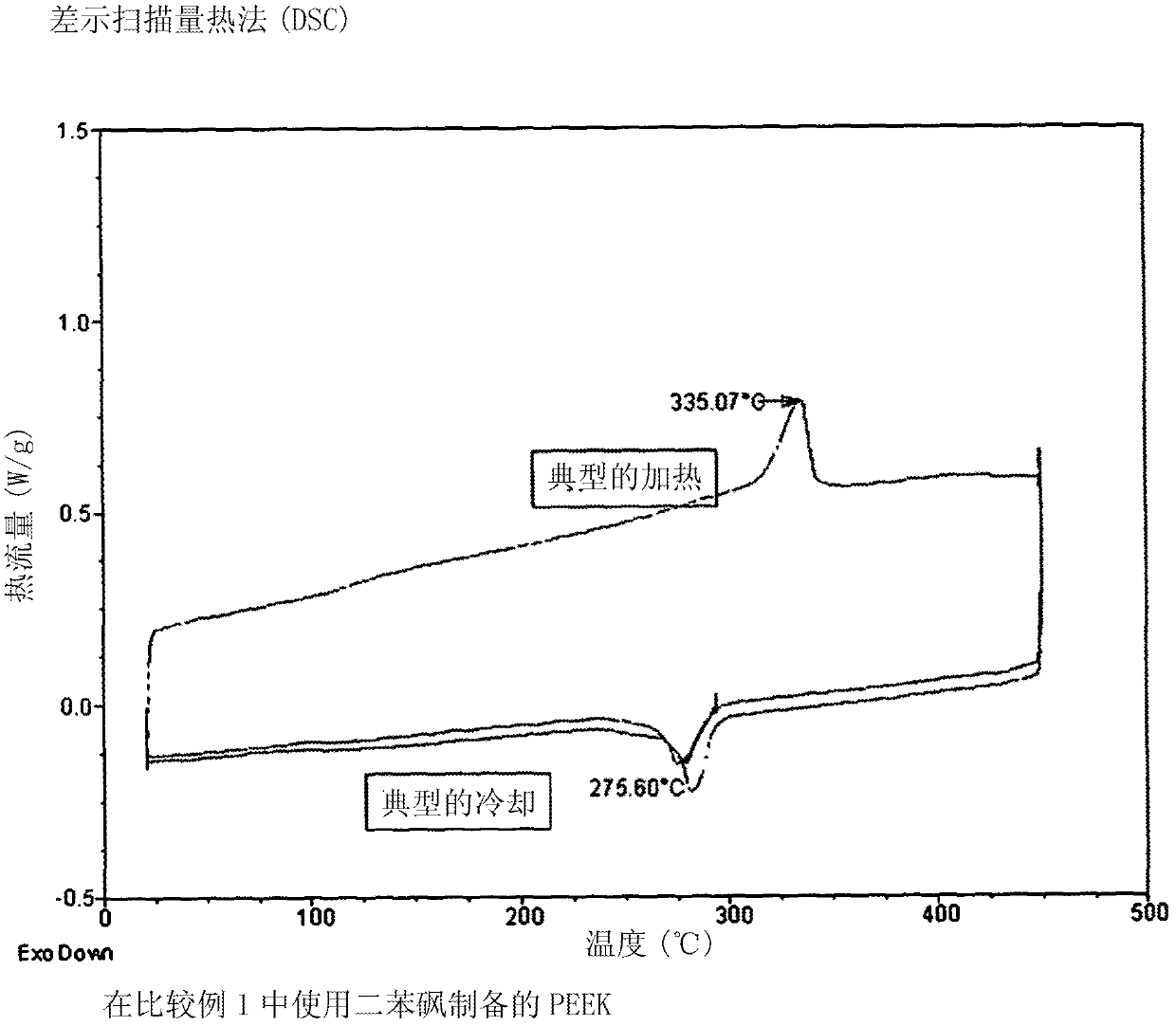
[0096] Hydroquinone (1.691 g, 15.36 mmol), 4,4-difluorobenzophenone (3.347 g, 15.34 mmol), ground potassium carbonate (2.144 g, 15.51 mmol) were purged using a Schlenk line through a vacuum / nitrogen cycle and ionic liquid bis{(trifluoromethyl)sulfonyl}amine 1-butyl-3-methylimidazole (15 mL). The mixture was stirred under a nitrogen atmosphere and heated at 200°C for 1 hour, then at 250°C for an additional 1 hour and finally at 320°C for an additional 1 hour. The obtained brown slurry was cooled to room temperature and mixed with acetone (40 mL). The resulting suspension was stirred vigorously. The resulting product powder was collected by filtration, washed with water (3 x 20 mL) and then with an acetone / methanol mixture (1:1 by weight, 3 x 20 mL). The product was next dried under a stream of air and then under high vacuum at 140 °C for 12 h to give a brown solid (3.684 g, 83%). The product was characterized by differential scanning calorimetry (DSC) under nitrogen at a h...
Embodiment 2
[0098] As in Example 1, a mixture of hydroquinone (1.652 g, 15.00 mmol), 4,4-difluorobenzophenone (3.273 g, 15.00 mmol) and ground potassium carbonate (2.103 g, 15.22 mmol) was made In the ionic liquid bis{(trifluoromethyl)sulfonyl}amine 1-butyl-3-methylimidazole as solvent (15mL). The reaction was carried out at a temperature of 200°C for 2 hours, and then at 250°C for a further 2 hours. The resulting white reaction product slurry was then treated the same as in Example 1 to give a white solid (4.090 g, 95% yield). The product was characterized as in Example 1 (m.p. = 329°C, RV = 1.32).
Embodiment 3
[0100] As in Example 1, a mixture of hydroquinone (1.003 g, 10.02 mmol), 4,4-dichlorobenzophenone (2.517 g, 10.02 mmol) and ground potassium carbonate (1.399 g, 10.12 mmol) was made In the ionic liquid bis{(trifluoromethyl)sulfonyl}amine 1-butyl-3-methylimidazole as solvent (10mL). The resulting brown product slurry was then treated the same as in Example 1 to give a white solid (2.150 g, 74% yield). The product was characterized the same as in Example 1 (RV = 1.23).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com