Method for preparing chemical compounds of interest by aromatic nucleophilic substitution
A technology of aromatics and compounds, applied in the field of chemical synthesis, can solve problems such as inability to handle and produce
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
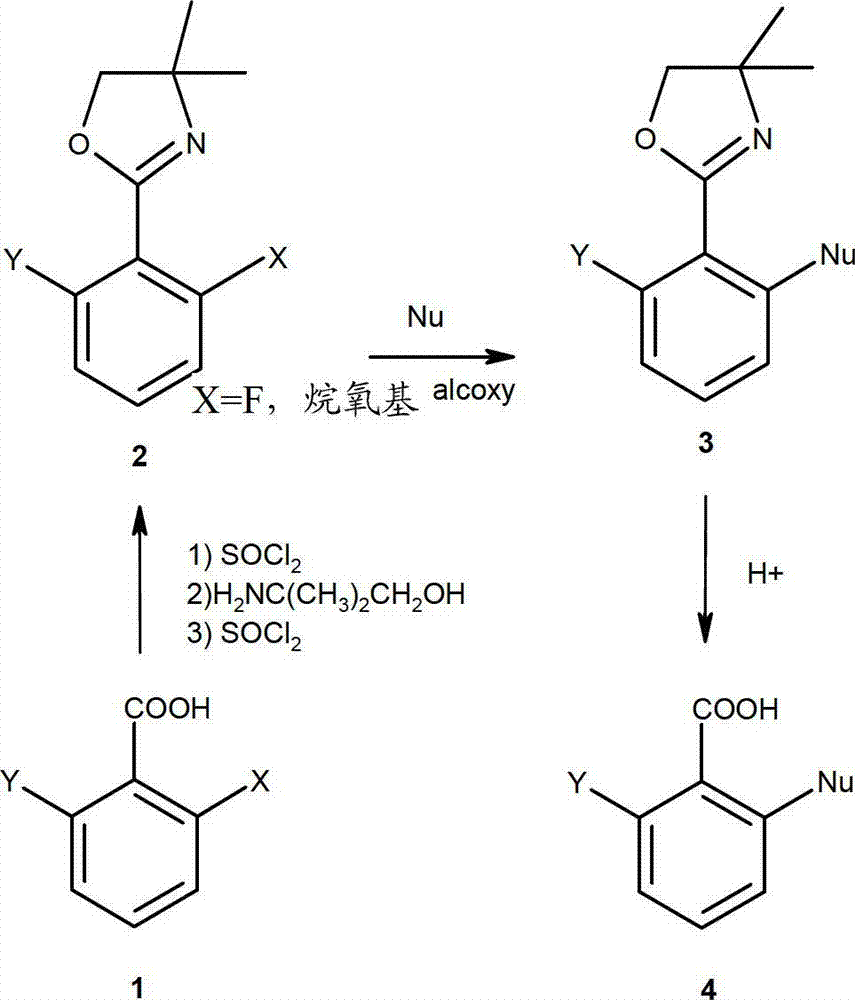
[0081] According to a first embodiment, Nu is not a substituted or unsubstituted amine, especially not an aniline derivative, more especially not 4-[2-(3,4, when the fluorine or chlorine atom is located in the ortho position of the acid function. -Dichlorophenyl)ethyl]aniline.
[0082] According to a second embodiment, Nu is not a substituted or unsubstituted amine when the fluorine atom is located ortho to the acid function.
[0083] According to one embodiment of the present invention, in compound (II), the leaving group (R1 or R2) is a fluorine or chlorine atom, and the nucleophile of the compound of general formula NuM is an aniline derivative. In this embodiment, according to the first aspect, the NuM compound is obtained according to the synthesis described below, with the proviso that NuM is not a reaction product between a nucleophile and a metal base selected from lithium hydride, sodium hydride , potassium hydride, calcium hydride, lithium diisopropylamide, lithium ...
Embodiment
[0128] All reactions were performed in anhydrous solvents under an inert atmosphere (Gordon, J.A.; Ford, R.A. The Chemist's Companion, Wiley J. and Sons, New York, 1972). THF was distilled over anhydrous THF GTS100 bench (Glass Technology). Alkyllithium derivatives are periodically titrated with N-benzylbenzamide (Burchat, A.F.; Chong, J.M.; Nielsen, N.J. Organomet. Chem. 1997, 542, 281).
[0129] S-butyllithium (1.4M solution in cyclohexane), n-butyllithium (1.6M solution in hexane), tert-butyllithium (1.7M solution in pentane) and phenyllithium (1.8M solution in dibutyl ether) were prepared by Sold by Acros Chemicals and Aldrich Chemical Company.
[0130] Ethylmagnesium bromide (3M in diethyl ether) and vinylmagnesium bromide (1M in THF) are sold by Acros Chemicals and Aldrich Chemical Company.
[0131] Amines in CaH 2 Distilled and stored under argon atmosphere.
[0132] proton 1 H (400MHz or 200MHz) and Carbon 13NMR spectra of C (50 MHz or 100.6 MHz) were recorded on...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com