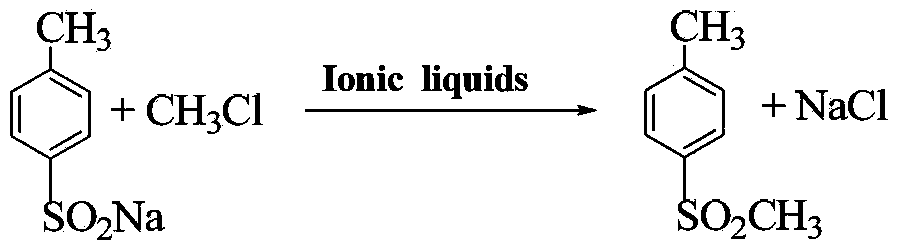
Method for preparing 4-methylsulfonyltoluene
A technology of thiamphenicol toluene and sodium toluene sulfinate, applied in the field of preparation of 4-thiamphenicol toluene, which can solve the problems of slow methylation rate, large loss of inorganic salt removal, long production cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
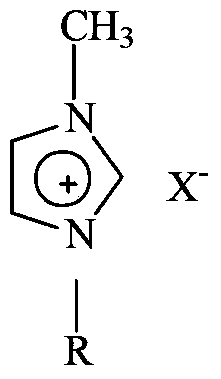
[0026] Dissolve a certain proportion of anhydrous sodium sulfite and sodium bicarbonate in water, stir evenly, and slowly add a certain amount of p-toluenesulfonyl chloride at 85°C. After the addition is completed, the reaction is kept for 1 hour, and the reaction is completed, cooling, suction filtration and drying to obtain crude sodium 4-methylbenzenesulfinate. Take 10g of 4-methylsulfonyl toluene and 100g of sodium 4-methylbenzenesulfinate (the content of sodium 4-methylbenzenesulfinate is measured by ultraviolet analysis, and the dosage is calculated based on the content. The same implementation is carried out in the following examples Example 1), 1 g of boron tetrafluoride 1-butyl-3-methylimidazole ionic liquid was added to the autoclave. The reaction kettle is connected to a methyl chloride cylinder. After the pressure stabilizes at 0.4Mpa, the temperature is raised to 80°C. After the reaction starts, the pressure drops to 0.2Mpa. Continue to keep the methyl chloride gas...
Embodiment 2
[0029] Take 20 g of 4-methylsulfonyl toluene, 100 g of sodium 4-methylbenzenesulfinate, and 2 g of boron tetrafluoride 1-butyl-3-methylimidazole ionic liquid, and put them into the autoclave. The reaction kettle is connected to a methyl chloride cylinder. After the pressure stabilizes at 0.5Mpa, the temperature is raised to 85°C. After the reaction starts, the pressure drops to 0.2Mpa. Keep the methyl chloride gas flowing. When the pressure is constant at 0.5Mpa, after reacting for 1 hour, Close the inflation valve. After the reaction, the reaction kettle is heated at 100°C and discharged. Add hot water and stir in the output mixture. When the water temperature is slightly cold, perform suction filtration, and then wash with a small amount of water. After cooling, white crystals are precipitated. The product is obtained after suction filtration and drying. 90.08g, the measured melting range: 87.3~87.9℃. The product was analyzed by gas chromatography with a purity of 98.1%.
Embodiment 3
[0031] Take 50 g of 4-methylsulfonyl toluene, 100 g of sodium 4-methylbenzenesulfinate, and 2 g of boron tetrafluoride 1-pentyl-3-methylimidazole ionic liquid, and add them to the autoclave. The reaction kettle is connected to a methyl chloride cylinder. After the pressure stabilizes at 0.4Mpa, the temperature is raised to 83°C. After the reaction starts, the pressure drops to 0.2Mpa. Keep the methyl chloride gas flowing. When the pressure is constant at 0.4Mpa, after reacting for 1 hour, Close the inflation valve. After the reaction, the reaction kettle is heated at 90°C and the material is discharged. Add hot water to the output mixture and stir. When the water temperature is slightly cold, suction filtration, and then wash it with a small amount of water. After cooling, white crystals are precipitated, and the product is obtained after suction filtration and drying. 85.98g, the measured melting range: 86~86.8℃. The product was analyzed by gas chromatography with a purity of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com