Sulfonated compound containing phosphinyl structure and preparation method thereof
A compound and sulfonation technology, applied in the chemical industry, can solve the problem of less performance characterization, and achieve the effects of comprehensive performance improvement, high proton conductivity, and enhanced interaction force
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
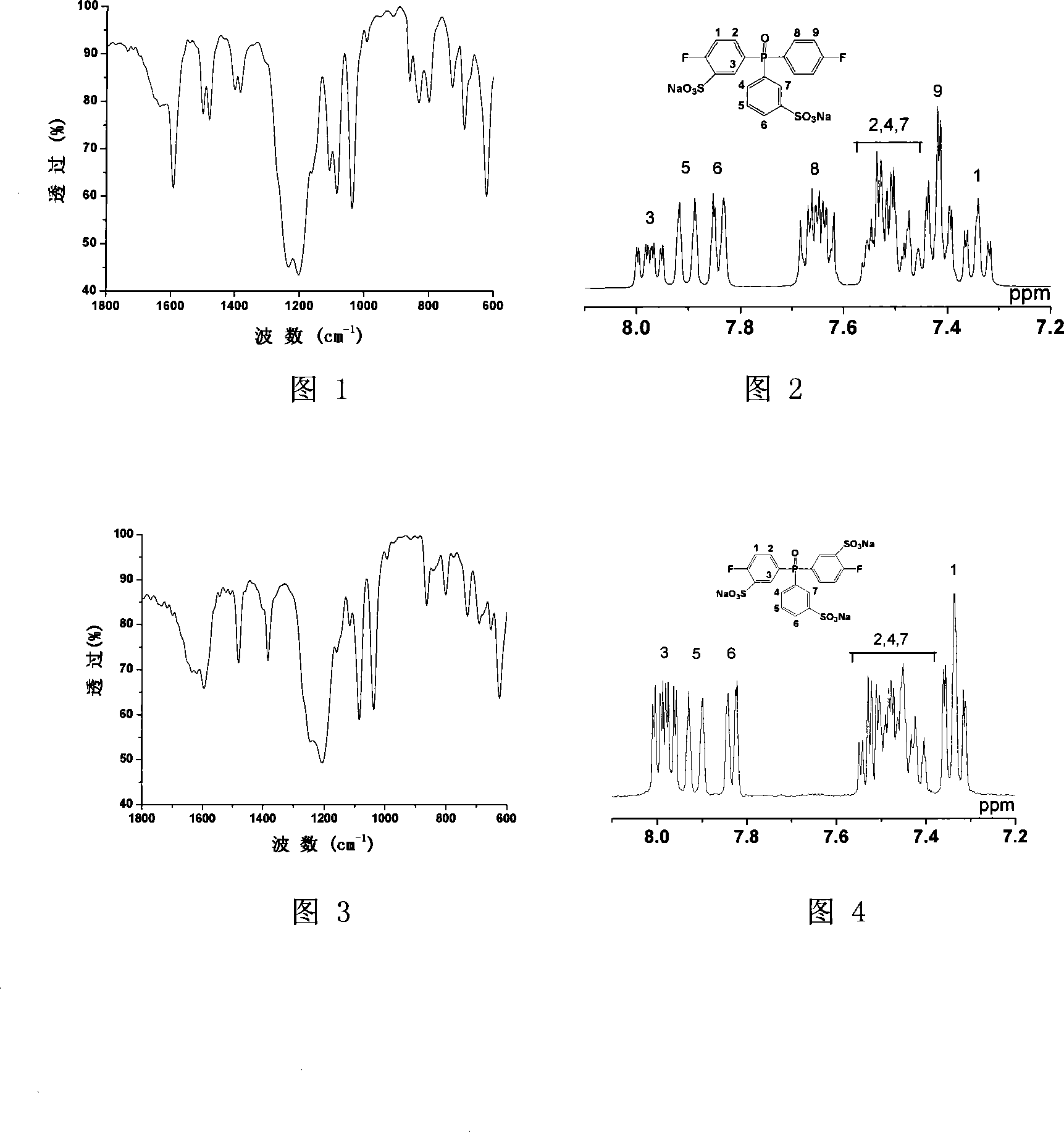
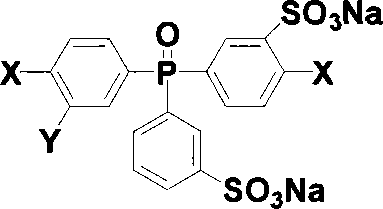
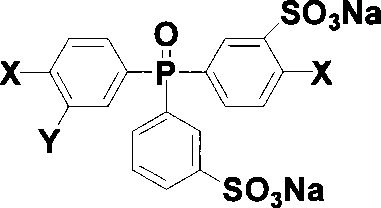
[0026] Add 5 grams of two (4-halophenyl) phenylphosphine oxides and 7.5 milliliters of 35% fuming sulfuric acid in a 50 milliliter three-neck round bottom flask equipped with mechanical stirring, react at 0 to 60 ° C for 3 hours, then The reaction was carried out at 90 and 110° C. for 2 hours and 8 hours, respectively. After cooling, the reaction solution was poured into a mixture of ice and water, salted out with sodium chloride, and the light red oily substance sank to the bottom, extracted with butanone, and the extract was evaporated to dryness by a rotary evaporator to obtain a crude product, which was dissolved in water. Neutralize with sodium hydroxide and then decolorize with activated carbon, evaporate water by rotary evaporator, use methanol / ethyl acetate as eluent for column separation, and vacuum dry to obtain the target product 3-sodium sulfonate-4-halobenzene Base-3'-sodium sulfonate phenyl-4"-halophenylphosphine oxide, the yield is 80%. The structure of the prod...
Embodiment 2
[0030] Add 3 grams of two (4-halophenyl) phenylphosphine oxides and 4.5 milliliters of 30% fuming sulfuric acid in a 50 milliliter three-necked round bottom flask equipped with mechanical stirring, react at 0 to 60 ° C for 2.5 hours, and then The reaction was carried out at 90 and 110° C. for 1 hour and 9 hours, respectively. After cooling, the reaction solution was poured into a mixture of ice and water, salted out with sodium chloride, and the light red oily substance sank to the bottom, extracted with butanone, and the extract was evaporated to dryness by a rotary evaporator to obtain a crude product, which was dissolved in water. Neutralize with sodium hydroxide and then decolorize with activated carbon, evaporate water by rotary evaporator, use methanol / ethyl acetate as eluent for column separation, and vacuum dry to obtain the target product 3-sodium sulfonate-4-halobenzene Base-3'-sodium sulfonate phenyl-4 "-halophenylphosphine oxide, yield 75%. Product structure obtain...
Embodiment 3
[0032] Add 10 grams of two (4-halophenyl) phenylphosphine oxides and 20 milliliters of 50% fuming sulfuric acid in a 100 milliliter three-necked round bottom flask equipped with mechanical stirring, react at 0 to 90 ° C for 4 hours, and then The reaction was carried out at 110 and 130° C. for 3 hours and 10 hours, respectively. After cooling, the reaction solution was poured into a mixture of ice and water, salted out with sodium chloride, and the light red oily substance sank to the bottom, extracted with butanone, and the extract was evaporated to dryness by a rotary evaporator to obtain a crude product, which was dissolved in water. Neutralize with sodium hydroxide and then decolorize with activated carbon, evaporate water by rotary evaporator, use methanol / ethyl acetate as eluent for column separation, and obtain the target product bis(3-sodium sulfonate-4- Halophenyl)-3'-sulfonate sodium phenylphosphine oxide, yield 70%. The structure of the product was obtained by infra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com