Method for producing optically active 2, 3-bisphosphinopyrazine derivative and method for producing optically active phosphine transition metal complex
a technology of phosphine and pyrazine, which is applied in the direction of group 5/15 element organic compounds, chemical instruments and processes, organic chemistry, etc., can solve the problems that the target isomer of a 2,3-bisphosphinepyrazine derivative having high optical purity cannot be obtained at good yield, and achieves high optical purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
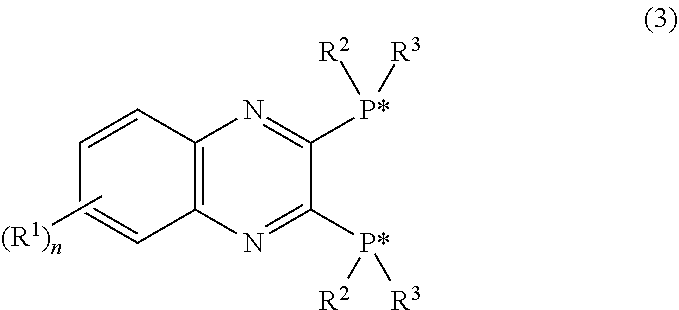
Synthesis of (R,R)-2,3-bis(tert-butylmethylphosphino)quinoxaline (a3)
[0072]
[0073]The air in a 300 mL four-neck flask which had been thoroughly dried was replaced with nitrogen, and then the flask was charged with 111.46 g (135.0 mmol) of a solution of 14.2% by mass (S)-tert-butylmethylphosphine-borane (a2) prepared above in tetrahydrofuran. The mixture was cooled to −10° C. under nitrogen atmosphere, and then 59.3 g of a 15% by mass n-butyllithium solution in hexane was added dropwise thereto over 1 hour. The mixture was aged for 1 hour at −10° C. to give solution B.
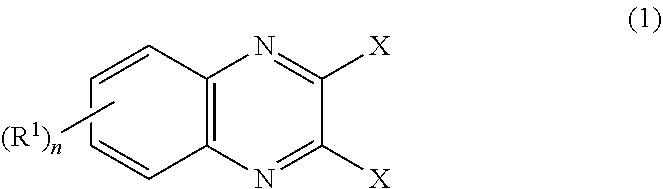
[0074]A 2,000 mL four-neck flask which had been thoroughly dried was separately prepared, and the air in the flask was replaced with nitrogen. Then the flask was charged with 8.97 g (45.0 mmol) of 2,3-dichloroquinoxaline (a1), tetrahydrofuran (81 ml), and 90 ml of N,N-dimethylformamide, and the mixture was cooled to −10° C. to give solution A.
[0075]Solution B was added to solution A at a constant rate over 40 minutes so ...
example 2
Synthesis of (R,R)-2,3-bis(tert-butylmethylphosphino)quinoxaline (a3)
[0081]Reaction was performed in the same manner as in Example 1 except for changing the amount of tetrahydrofuran to 81 ml and the amount of N,N-dimethylformamide to 45 ml in solution A to give (R,R)-2,3-bis(tert-butylmethylphosphino)quinoxaline (a3) (yield 69.4%). This crystal had a purity of 99.2% as measured by 31P NMR, and an optical purity of 99.5% ee or more.
example 3
Synthesis of (R,R)-2,3-bis(tert-butylmethylphosphino)quinoxaline (a3)
[0082]Reaction was performed in the same manner as in Example 1 except for changing the amount of tetrahydrofuran to 81 ml and the amount of N,N-dimethylformamide to 20 ml in solution A to give (R,R)-2,3-bis(tert-butylmethylphosphino)quinoxaline (a3) (yield 64.6%). This crystal had a purity of 99.1% as measured by 31P NMR, and an optical purity of 99.5% ee or more.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| reaction temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com