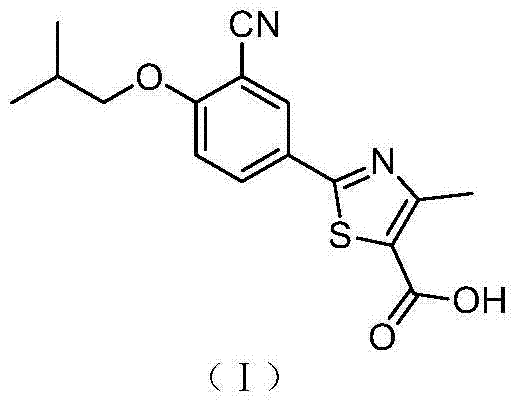
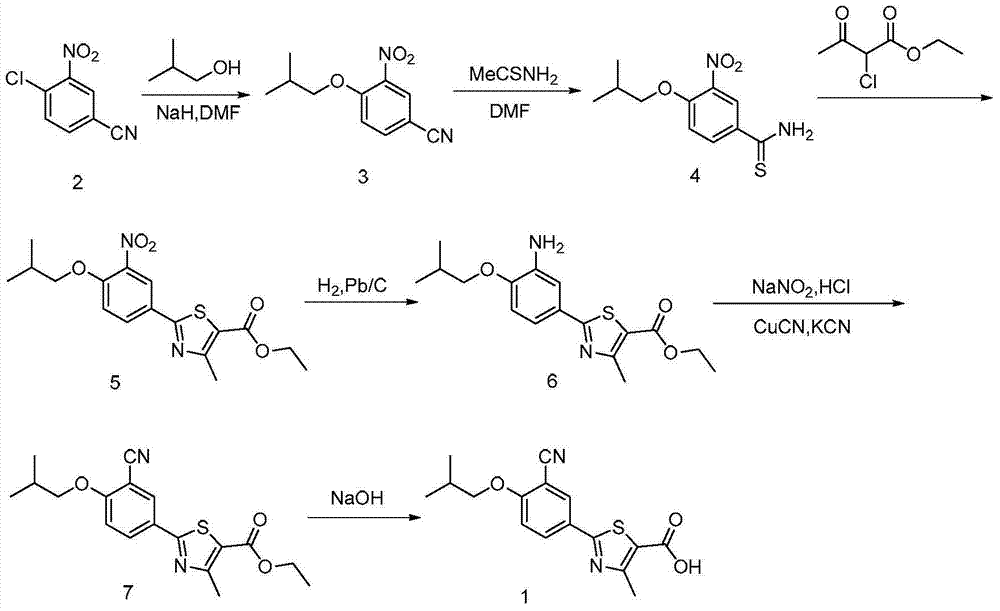
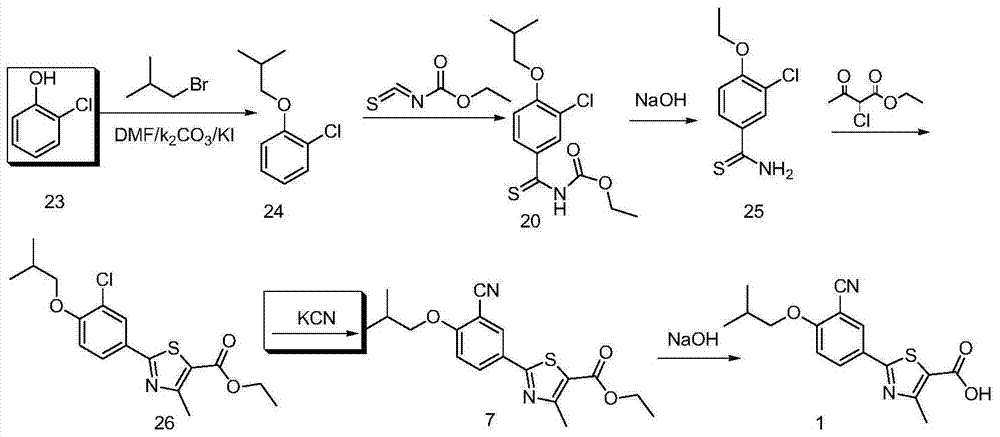
A kind of synthetic method of febuxostat
A febuxostat compound technology, applied in the field of febuxostat synthesis, can solve the problems of low industrialization feasibility, serious environmental pollution, and difficult wastewater treatment, so as to avoid the problem of unstable reaction, simple waste liquid treatment, harm reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] A preparation method of febuxostat, comprising the steps of:
[0051]
example 1
[0053] 1) Preparation of ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride:
[0054] Inject 250L of absolute ethanol into a dry and clean 500L enamel reaction kettle equipped with a thermometer and agitator, start stirring, add 50kg of 4-hydroxythiobenzamide into the reaction kettle from the solid feeding port, continue stirring and turn on the external The bath is heated up. During the heating process, 59kg of ethyl 2-chloroacetoacetate is pumped into a 100L dry and clean high-level tank. When the system temperature rises to about 50°C, the system dissolves and continues to heat up to 70°C. Add 20kg of ethyl 2-chloroacetoacetate, when the system is turbid (the system is turbid because the reflux reaction is violent, and a large amount of solids are washed out, at this time, a constant stirring should be ensured) immediately stop the dropwise addition, and stop the external bath heating at the same time, until the reflux is stable (The process lasts about ...
example 2
[0070] 1) Preparation of ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate hydrochloride:
[0071] Inject 260L of absolute ethanol into a dry and clean 500L enamel reaction kettle equipped with a thermometer and agitator, start stirring, add 50kg of 4-hydroxythiobenzamide into the reaction kettle from the solid feeding port, continue stirring and turn on the external The temperature of the bath is raised. During the heating process, 60kg of ethyl 2-chloroacetoacetate is pumped into a 100L dry and clean high-level tank. When the system temperature rises to about 50°C, the system dissolves, and when the temperature continues to rise to 72°C, it begins to drip Add 20kg of ethyl 2-chloroacetoacetate, when the system is turbid (the system is turbid because the reflux reaction is violent, and a large amount of solids are washed out, at this time, a constant stirring should be ensured) immediately stop the dropwise addition, and stop the external bath heating at the same time, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com