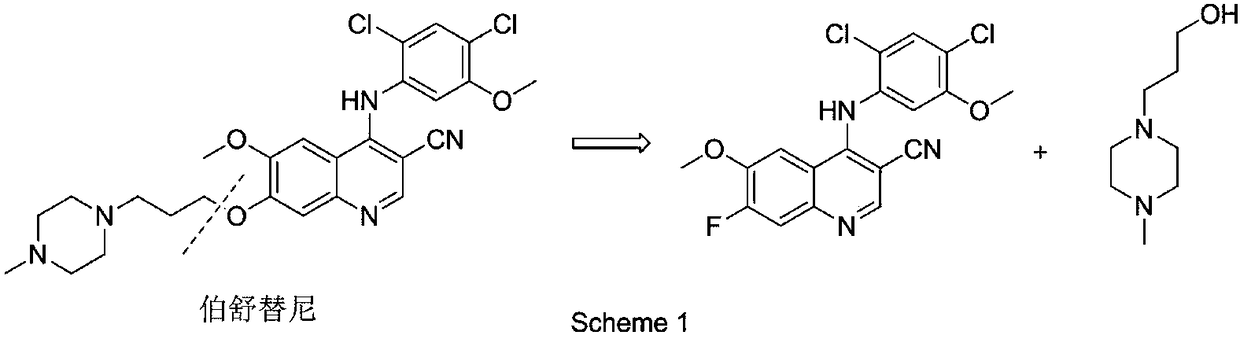
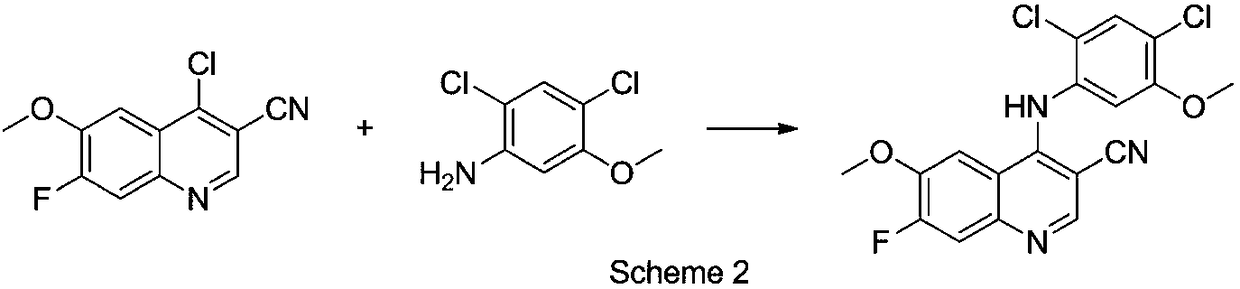
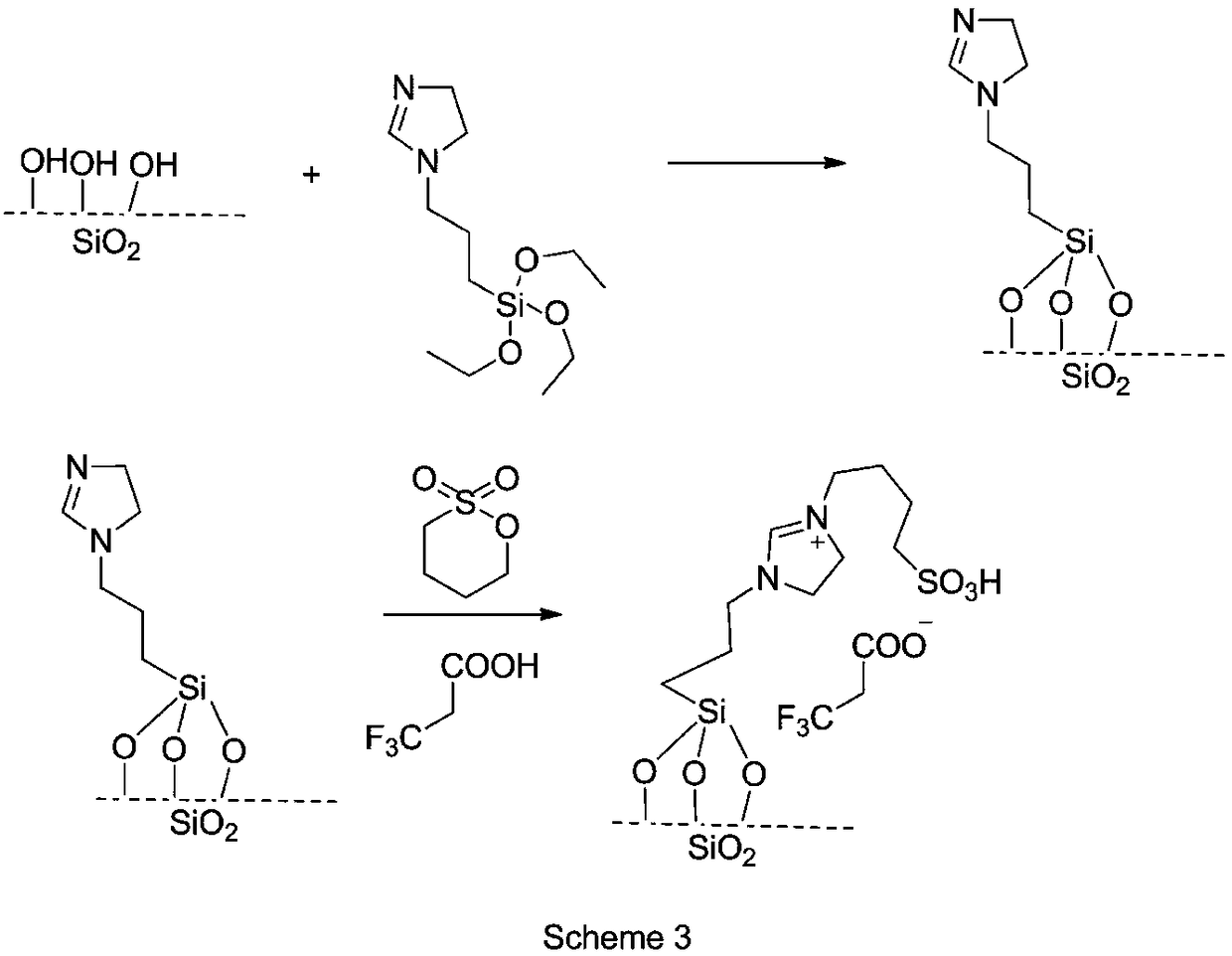
Preparation method of intermediate for leukemia treatment medicines
An intermediate and leukemia technology, which is applied in the field of preparation of intermediates for treating leukemia drugs, can solve the problems of high production cost and low yield, and achieve the effects of uniform particle size distribution, high yield, and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of silica-supported acidic ionic liquids:
[0034] 1) Disperse 5.0g of nano-silica in 100ml of chloroform and stir evenly, then add 2.74g of N-[3-(triethoxysilyl)propyl]-4,5-dihydroimidazole for reflux reaction for more than 12h, and cool down to Filter at room temperature, rinse with ammonia water and acetone in turn, and dry to obtain dihydroimidazole-modified silica gel;
[0035] 2) Put 5.0 g of dihydroimidazole-modified silica gel and 1.36 g of 1,4-butane sultone in 200 ml of acetone to react at room temperature for 24 hours, then add 1.14 g of trifluoroacetic acid or 1.50 g of trifluoromethanesulfonic acid or p-toluene 1.72 g of sulfonic acid was refluxed for 2-3 days, cooled to room temperature, filtered, washed with n-heptane and dried to obtain an acidic ionic liquid supported on silica gel.
[0036] The silica-supported acidic ionic liquids prepared from trifluoroacetic acid, trifluoromethanesulfonic acid and p-toluenesulfonic acid are respectively...
Embodiment 2
[0038] With the prepared IL / TFA@SiO 2 、IL / TfO@SiO 2 and IL / Ts@SiO 2 As a catalyst for catalytic performance evaluation:
[0039] Add 4-chloro-6-methoxy 7-fluoro-quinoline-3-carbonitrile (4.72g, 20mmol), 2,4-dichloro-5-methoxyaniline (4.61g, 24mmol) into the reaction flask ), stirred and dissolved in 80ml dimethyl sulfoxide, then added catalyst (0.50g, ~10.6wt%) to stir and disperse evenly, heated to 90-100°C for reaction, HPLC detection, 4-chloro-6-methanol in the reaction liquid Stop the reaction after the concentration of oxy-7-fluoro-quinoline-3-carbonitrile no longer drops, cool down to room temperature, filter and remove the acidic ionic liquid loaded on silica gel to obtain the filtrate; add the filtrate dropwise to 300ml saturated aqueous sodium bicarbonate solution at room temperature Crystallize, stir for 20-30min, filter and dry to constant weight to obtain 4-(2,4-dichloro-5-methoxyphenylamine)-7-fluoro-6-methoxyquinoline-3-carbonitrile Solid; 4-(2,4-dichloro-5-m...
Embodiment 3
[0045] After determining the acidic ionic liquid IL / TFA@SiO supported on silica gel 2 After being used as a catalyst, the molar ratio of the solvent and its 4-chloro-6-methoxyl 7-fluoro-quinoline-3-carbonitrile to 2,4-dichloro-5-methoxyaniline in the present invention, the amount of catalyst Optimized, the method is as follows:
[0046] Add 4-chloro-6-methoxy 7-fluoro-quinoline-3-carbonitrile (4.72g, 20mmol), 2,4-dichloro-5-methoxyaniline (20-30mmol) into the reaction flask , stir and dissolve in 80ml of solvent, then add catalyst (50mg-1.41g, 1.0wt%~30wt%), stir and disperse evenly, heat up to 90-100°C (solvent reflux reaction with boiling point lower than 90°C) for reaction, HPLC detection, Stop the reaction after the concentration of 4-chloro-6-methoxy 7-fluoro-quinoline-3-carbonitrile in the reaction solution no longer drops, cool down to room temperature, filter and remove the acidic ionic liquid loaded on silica gel to obtain the filtrate; Add dropwise to 300ml saturat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com