Organic ligand and Pd/Pb-based bi-metal organic framework as well as synthetic method and application thereof
A technology of organic framework and organic ligand, which is applied in the field of catalyst preparation, can solve the problems of excessive phosphine ligand usage, non-recyclable catalyst, and air sensitivity, and achieve the effects of less catalyst usage, easy recycling, and mild reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Preparation of Pd carbene metal organic ligand L
[0056] Concrete preparation steps are as follows:
[0057] (1)N 2 Under protection, methyl 4-bromobenzoate (4.3g, 20mmol), imidazole (2.04g, 30mmol), cesium carbonate (19.6, 60mmol), cuprous iodide (0.19g, 1mmol) were added to a three-necked flask, Add dry 100mL DMF, stir and heat to 130°C. After the reaction is monitored by TLC, pour it into 200mL saturated saline, extract with 200mL×3 dichloromethane, combine the organic phases, and wash with saturated saline three times, and wash the organic phase with anhydrous sulfuric acid Dry over magnesium, filter, remove the solvent under reduced pressure, and separate and purify by column chromatography to obtain 3.15 g of white solid with a yield of 78.00%.
[0058]
[0059] (2) Intermediate A (5 mmol, 1.01 g) was placed in a 100 ml single-necked bottle, 10 ml of dibromomethane was added, and heated to reflux overnight. According to TLC tracking, after the...
Embodiment 2
[0064] Embodiment 2: the synthesis of Pd / Pb-MOF
[0065] The organic ligand L (6.80 mg, 0.01 mmol) prepared in Example 1, lead nitrate (0.04 mmol, 13.3 mg), oxalic acid (1 mmg, 0.01 mmol) were dissolved in 2 mL of ethanol: water = 1:3 mixed solvent, Put it in a 5ml small test tube, keep the temperature at 150°C for 72 hours, and cool it down to room temperature after 50 hours, to obtain colorless blocky crystal {[Pb 4 (C 42 h 28 N 8 o 8 Pd)(C 2 o 4 )Br 4 ]}, yield 4.2 mg, yield 20% (based on L).
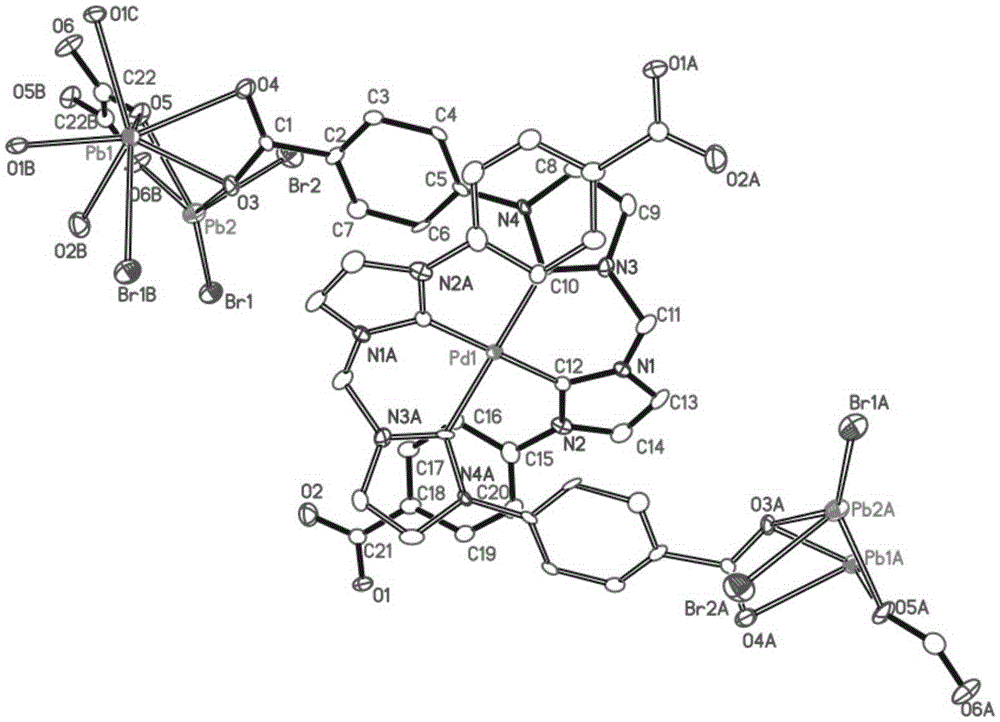
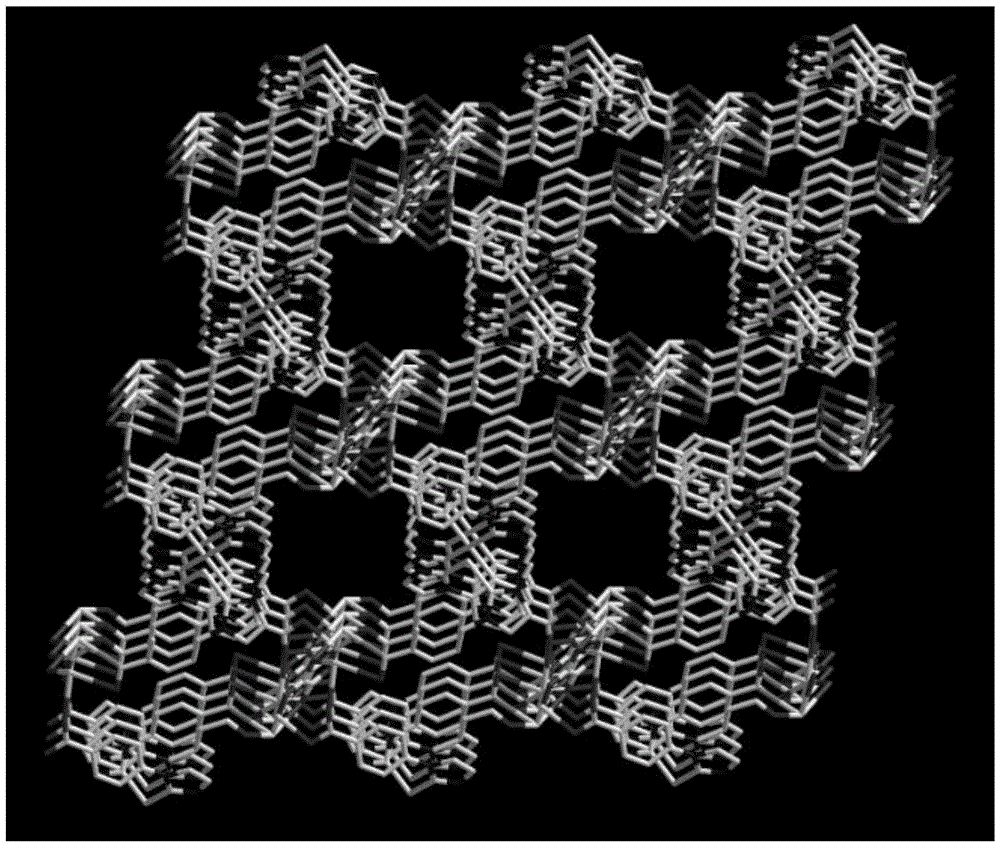
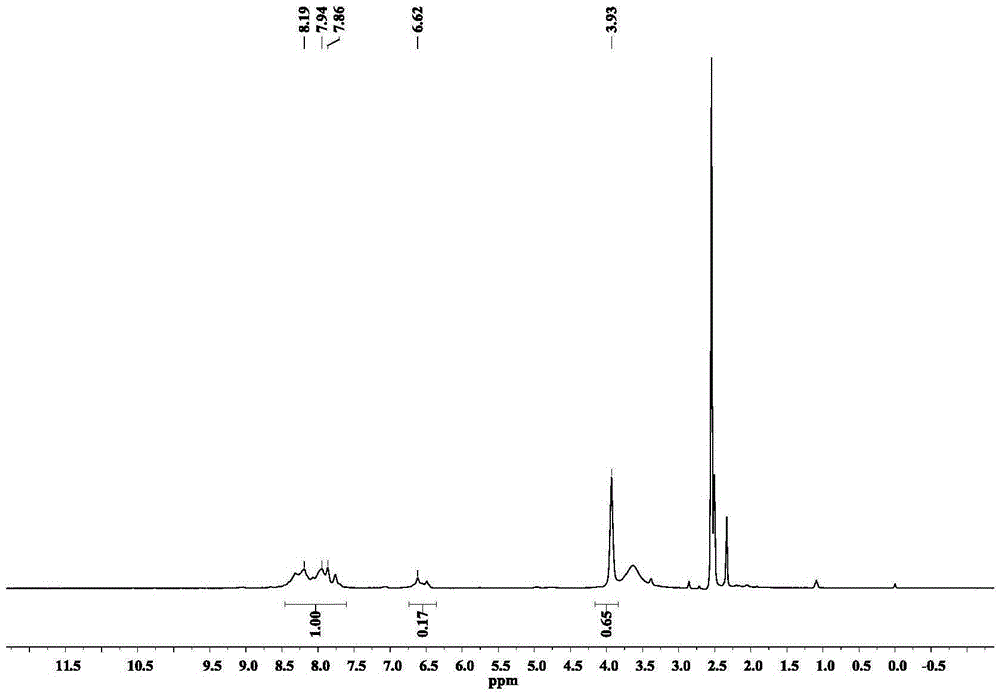
[0066] We characterized the compound by IR, TGA, the results are shown in Figure 5 with Image 6 , the single crystal structure of Pd / Pb-MOF is shown in figure 1 As shown, the three-dimensional structure of Pd / Pb-MOF is as follows figure 2 shown.
experiment example 3
[0067] Experimental example 3: Pd / Pb-MOF catalyzes the self-coupling of benzyne to generate 9,10-triphenylene. The reaction equation is as follows:
[0068]
[0069] 2-(Trimethylsilyl)phenyltrifluoromethanesulfonate (0.5mmol, 121 μL), cesium fluoride (1.5mmol, 228mg), 2ml of acetonitrile were added to a 25ml single-necked round bottom flask, and then 12mg of catalyst compound was added, Stir, heat to 60°C, monitor the reaction progress by TLC, after two hours the reaction is over, centrifuge quickly to recover the catalyst, the product is extracted with dichloromethane, separated by column chromatography, the calculated yield is 72%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com