Organic phosphine compound with sulfonyl functional group, as well as preparation method and application thereof
A technology of sulfonyl functional and phosphine compounds, which is applied in the field of organic phosphine compounds, can solve the problems of low synthesis efficiency, cumbersome operation, and difficulty in obtaining functional group compounds, and achieve the effect of accelerated discovery and optimization, and good technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
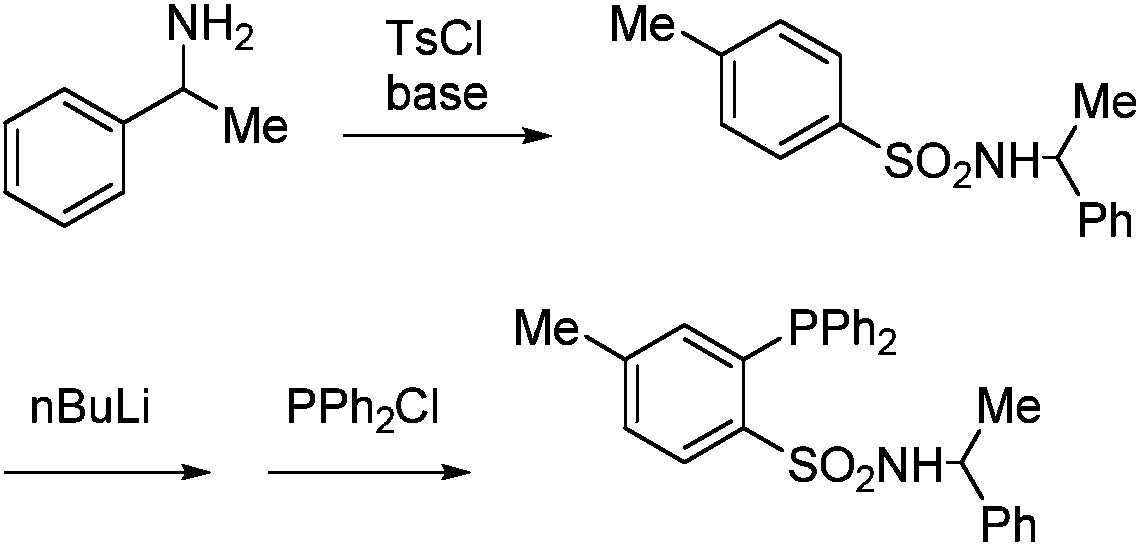
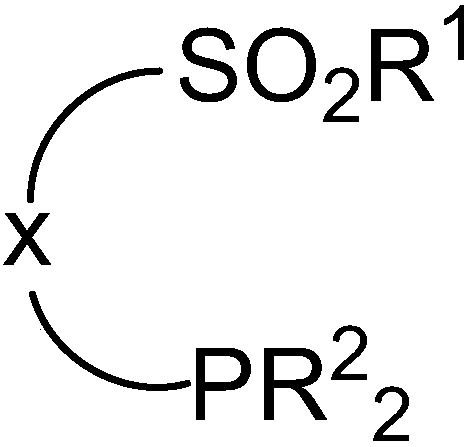
[0024] The preparation method of the above-mentioned organic phosphine compound with sulfonyl functional group comprises the following steps: a) reacting sulfonyl chloride with a reagent with a leaving group, introducing R 1 Functional group; b) construct carbon-phosphine bond through nucleophilic substitution reaction or coupling reaction, and introduce phosphine-containing group.
[0025] The above organic phosphine compounds having sulfonyl functional groups can be applied to the preparation of sulfonamide phosphine-containing compounds. Sulfonamide-type phosphine-containing compounds can be constructed by directly reacting nitrogen-containing nucleophiles with sulfonyl functional groups (see below). This route has mild reaction conditions and good functional group compatibility, which provides a quick way to construct a ligand library, which is much better than the prior art, and the obtained sulfonamide phosphine-containing compounds can be used as catalysts. Preferably,...
Embodiment 1
[0028] Embodiment 1 benzenesulfonyl chloride is raw material synthesis compound III
[0029]
[0030] 1. Pentafluorophenol 2-iodo-5-methylbenzenesulfonate (II) (R=I)
[0031] Pentafluorophenol and dry dichloromethane (-0.13-1 M) were added to the reaction flask, and triethylamine (2-3 equiv) was added dropwise to the solution. After stirring at room temperature for 30-120 minutes, a solution of compound III in dichloromethane (1-2 equiv, 0.5M) was added dropwise to the mixture. After the addition was complete, the reaction continued to stir at room temperature for 12-48 hours. Dichloromethane was then added for dilution, and the organic phase was washed sequentially with 1N aq HCl, 2N aq. NaOH and brine. After the obtained organic phase was dried over anhydrous sodium sulfate, the solvent was removed to obtain the white target product II with a yield of 85-90%. The test result is: 1 H NMR (400MHz, CDCl 3 )δ: 2.41(s, 3H), 7.20(d, J=8Hz, 1H), 7.87(s, 1H), 7.76(t, J=8Hz, ...
Embodiment 2
[0036] Example 2 Application of compound IV to construct sulfonamide phosphine-containing compound V
[0037]
[0038] The preparation method of compound IV is the same as that of compound III in Example 1, except that the methyl group on the benzene ring is replaced by a methoxy group.
[0039] Add triethylamine (2-4 equivalents), 1-phenylethylamine (1-2 equivalents) and 4-dimethylaminopyridine (0.1-0.5 equivalents) to IV acetonitrile solution (1-2M), then mix the mixture After stirring for 12-24 hours, the reaction temperature ranged from room temperature to reflux. After the reaction, the acetonitrile was removed, and the obtained residue was purified by column chromatography to obtain the target product V in the form of white powder.
[0040] The test result is: 1 H NMR (400MHz, CDCl 3 )δ: 1.57(d, J=6.9Hz, 3H), 3.86(s, 3H), 4.52(p, J=6.8Hz, 1H), 6.94-7.35(m, 16H), 7.60(t, J=3.0 Hz,1H); 13 C NMR(101MHz,CDCl3)δ:160.00,146.99,146.73,141.97,137.76,136.25,136.19,135.75,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com