Cyclotriphosphazene derivative containing sulfydryl group and preparation method thereof
A technology for cyclotriphosphazene and derivatives, which is applied in the field of cyclotriphosphazene derivatives and their preparation, and phosphazene derivatives, can solve the problems of few applications, achieve good repeatability, mild reaction conditions, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
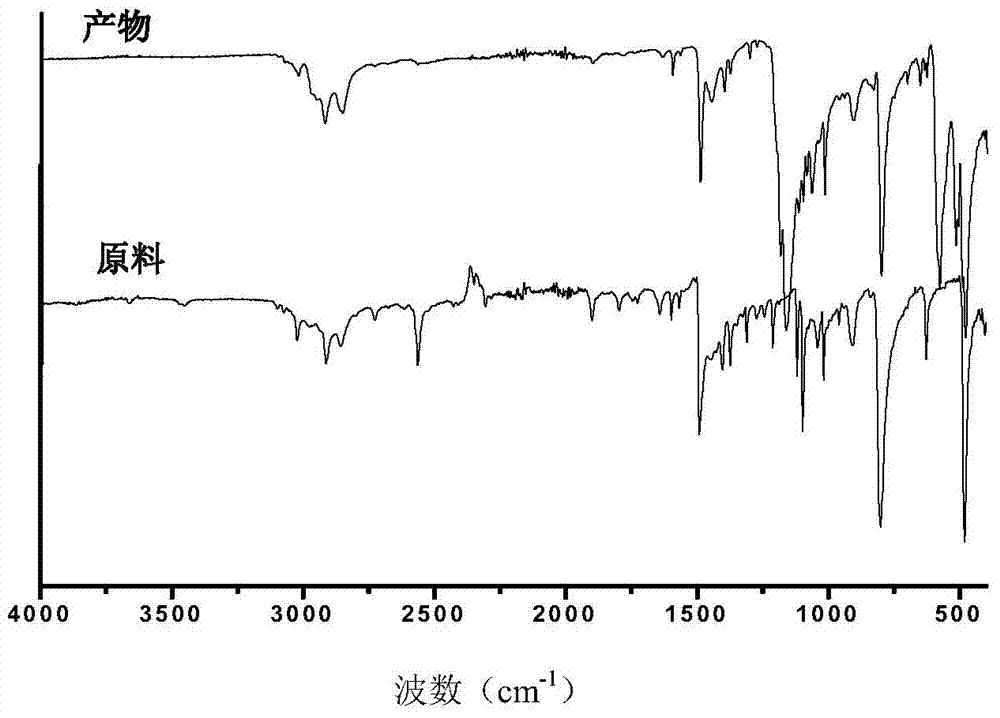
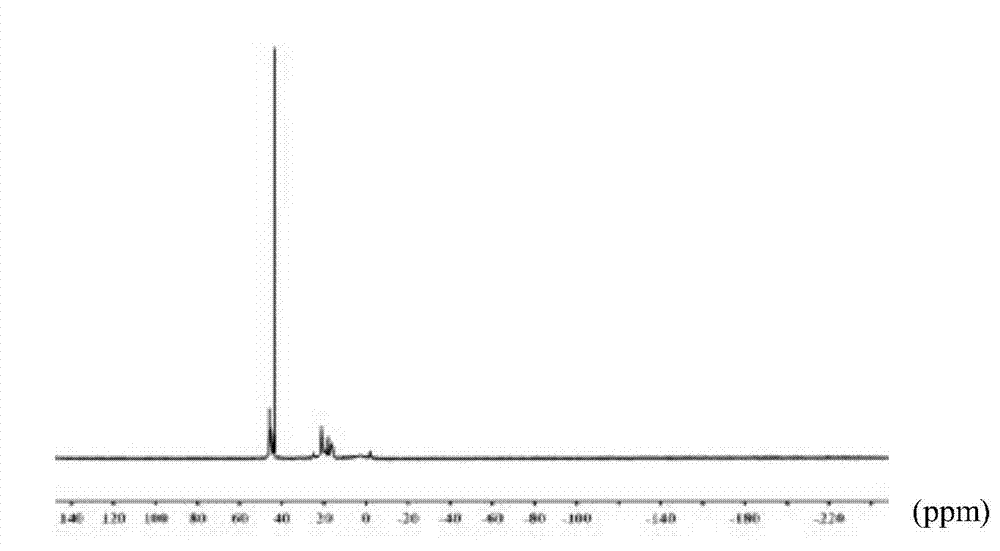
[0027] Dissolve 5.4g of thiophenol in 25ml of tetrahydrofuran, stir magnetically to fully dissolve, then add 1.05g of sodium hydride, slowly heat to 45°C, and drop 1.74g of hexachlorocyclotriphosphazene into the above tetrahydrofuran solution, at 45 Under the condition of ℃, react for 20h. After the reaction, the obtained liquid was suction-filtered to remove by-products, and vacuum rotary evaporation was used to remove tetrahydrofuran and excess raw materials in the system. Finally, the target product was a light yellow-green liquid with a pungent odor. The productive rate is 82.6%; The spectrogram data of five and six substituted cyclotriphosphazene derivatives containing mercapto groups are:
[0028] FT-IR (cm -1 ): 2500—2600 (Sulfhydryl), 3000—3100 (C=C), 1440—1510 (C-H) 1163 (P=N). 31 P NMR (CDCl 3 , δ, ppm, TMS): 40—50 (6P, -P-S-), 18—22 (1P, -P-Cl).
Embodiment 2
[0030] Dissolve 5.4g of thiophenol in 25ml of tetrahydrofuran, stir magnetically to fully dissolve, then add 1.05g of sodium hydride, slowly heat to 55°C, and drop 1.74g of hexachlorocyclotriphosphazene into the above tetrahydrofuran solution, at 55 Under the condition of ℃, react for 20h. The liquid obtained after the reaction was filtered to remove by-products, vacuum rotary evaporation to remove tetrahydrofuran and excess raw materials in the system, and finally the target product obtained was a light yellow-green liquid with a pungent smell. The yield is 83.2%;
Embodiment 3
[0032] Dissolve 5.4g of thiophenol in 25ml of tetrahydrofuran, stir magnetically to fully dissolve, then add 1.05g of sodium hydride, slowly heat to 65°C, and drop 1.74g of hexachlorocyclotriphosphazene into the above tetrahydrofuran solution, at 65 Under the condition of ℃, react for 20h. The liquid obtained after the reaction was filtered to remove by-products, vacuum rotary evaporation to remove tetrahydrofuran and excess raw materials in the system, and finally the target product obtained was a light yellow-green liquid with a pungent smell. The yield is 84.6%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com