Novel triterpenoid saponin compound and application in pest control thereof
A technology of triterpene saponins and compounds, which is applied in the preparation of pentacyclic triterpene glycosides and the application field of diamondback moth control, can solve the problems of separation and identification of active compounds, achieve good application prospects, simple operation, and low toxicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of general formula (1) compound
[0022] Use pomegranate with a dry weight of 9kg as the raw material, soak and extract with 95% ethanol at room temperature three times after crushing, each time for 2 days, then heat and reflux with 50% ethanol for three times, each time for 6 hours, the extracts are combined, concentrated under reduced pressure to obtain extract. The extract was suspended and dissolved in water to obtain a 3L solution, and then extracted with equal volumes of petroleum ether, ethyl acetate and n-butanol, each three times, and the n-butanol extract was concentrated under reduced pressure to obtain a crude extract (170 g). The extract was subjected to macroporous adsorption resin column (D101) chromatography, eluted with water, 30%, 60%, and 95% ethanol aqueous solution in sequence, and concentrated under reduced pressure respectively 60% and 95% ethanol aqueous solution to obtain two parts of samples 35g and 10g.
[0023]...
Embodiment 2
[0024] Embodiment 2: Structural identification and data assignment of the compound of general formula (1)
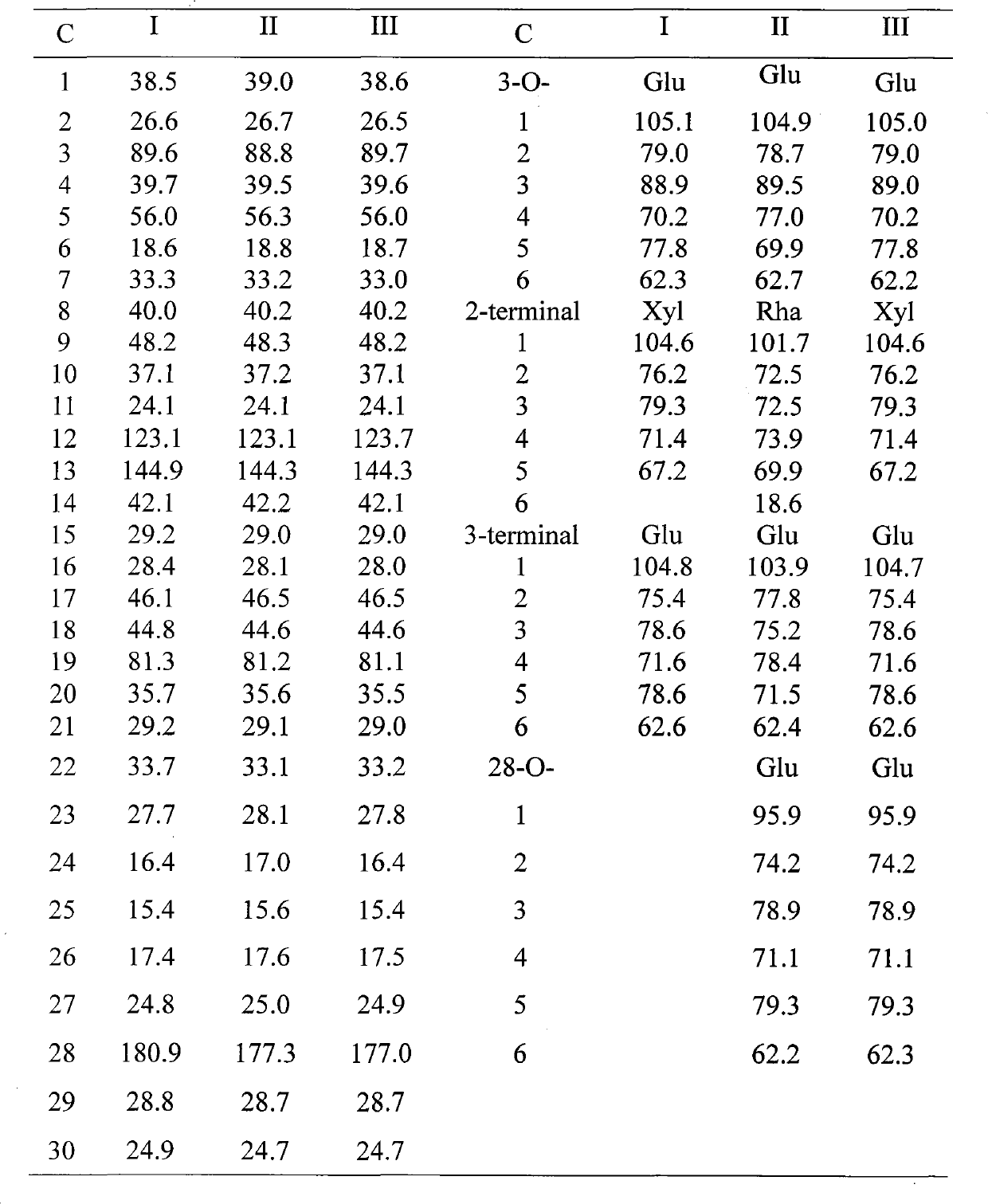
[0025] High resolution mass spectrum HRESI-MS m / z 951.4938[M+Na] of compound I in general formula (1) + Deduce its molecular formula as C 47 h 76 o 18 ; Through hydrochloric acid hydrolysis reaction, after TLC thin-layer chromatography, develop color with sulfuric acid chromogen and compare with the standard sample, it is determined that the sugar chain of the compound contains glucose and xylose; 1 H NMR and 13 C NMR can see the end group carbon signal of 3 sugar molecules in the compound [δ H 4.83(d, J=7.7Hz), 5.37(d, J=7.9Hz), 5.60(d, J=7.7Hz); δ C 105.1, 104.8, 104.6], from the methylene δ on the carbon spectrum C 67.2 Determine that one of the three molecular sugars is xylose and the other two are glucose. There are seven single-peak methyl proton signals in the high-field region of the hydrogen spectrum (δ H1.28, 1.07, 0.84, 1.03, 1.68, 1.20, 1.12); olefini...
Embodiment 3
[0030] Embodiment 3: Antifeedant activity test of punicalagin I-III to diamondback moth
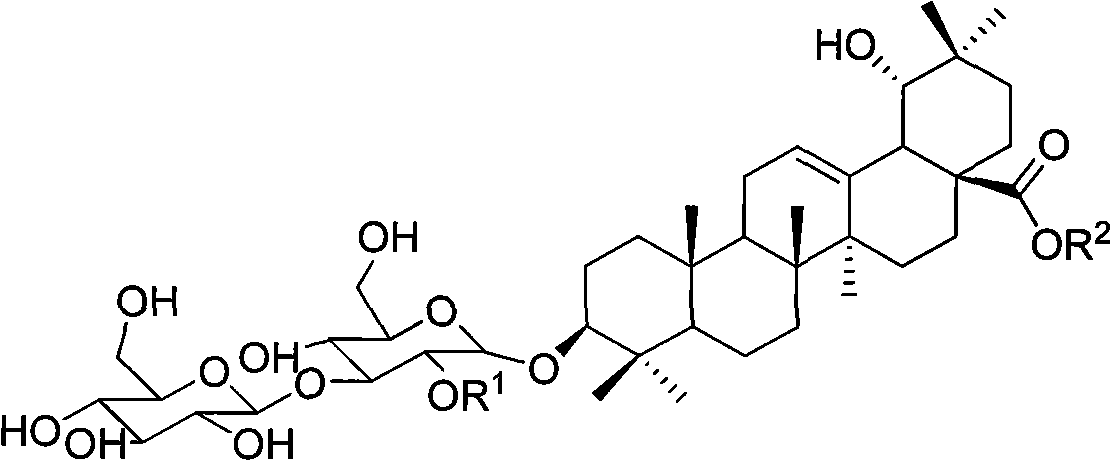
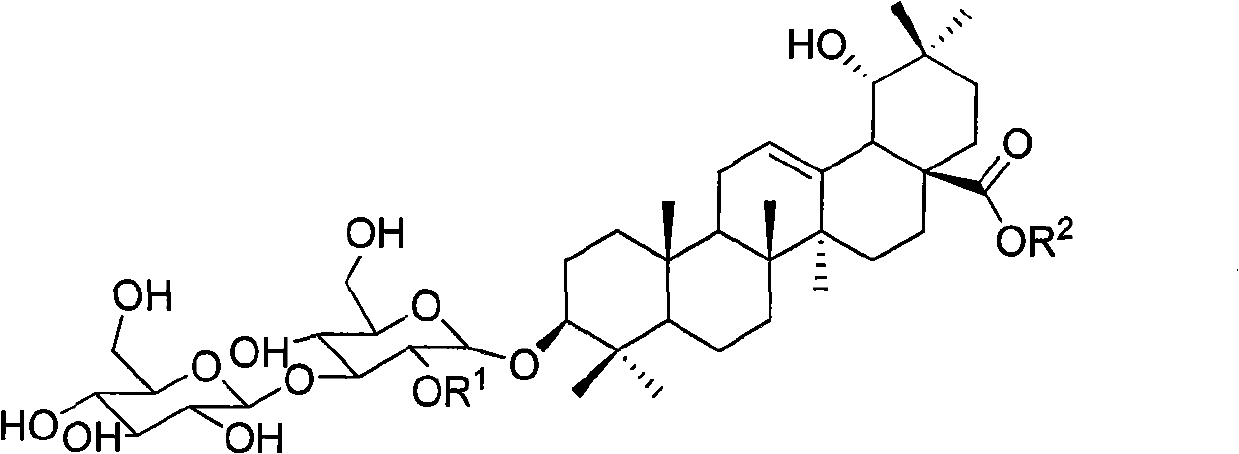
[0031] The structures of the three compounds are
[0032]
[0033] I R 1 = XylR 2 =H
[0034] II R 1 = Rha R 2 =Glu
[0035] III R 1 = XylR 2 =Glu
[0036] In this experiment, the Plutella xylostella population was raised in an artificial climate chamber (temperature: 25±1°C, humidity: 75±10%, photoperiod 14:10h (L:D)), and cabbage (Brassica oleraceae) was planted in Zhejiang Academy of Agricultural Sciences In the greenhouse of the Institute of Plant Protection and Microbiology, no pesticides were sprayed.
[0037] The antifeedant activity test adopts the leaf disk method: the sample compound is dissolved with 0.05% Tween-80 aqueous solution, and is prepared into an aqueous solution with a concentration gradient of 0.1, 1, 10, 100 and 1000 ppm, and a blank control with 0.05% Tween-80 aqueous solution. Use a puncher (diameter 1.5cm) to prepare round cabbage leaf discs, soak t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap