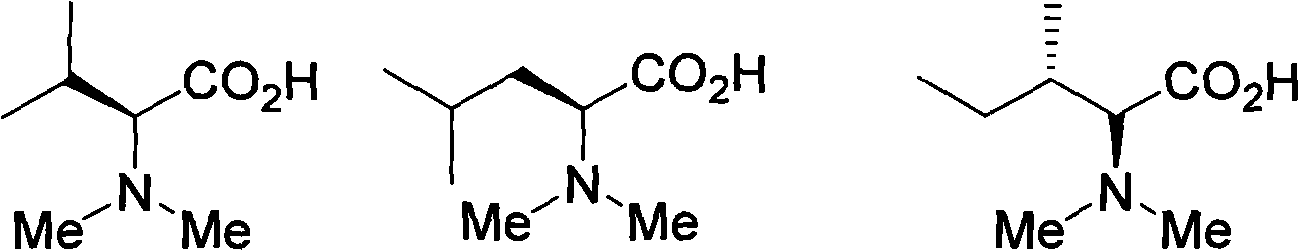
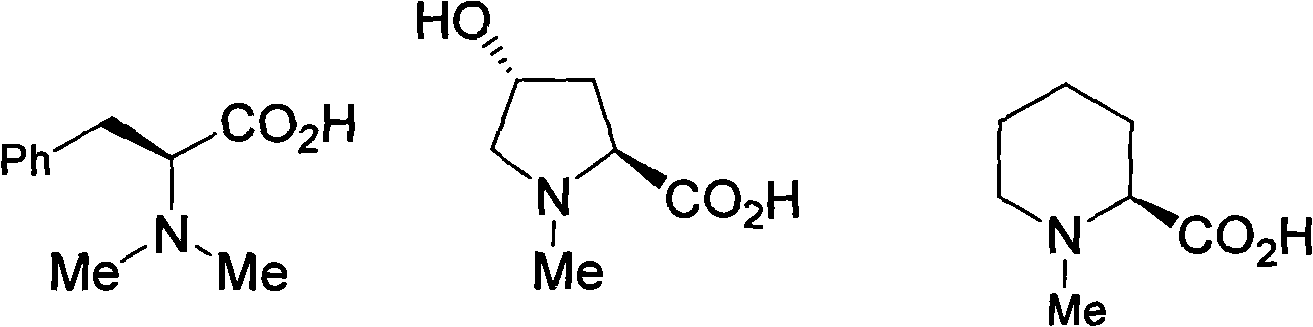
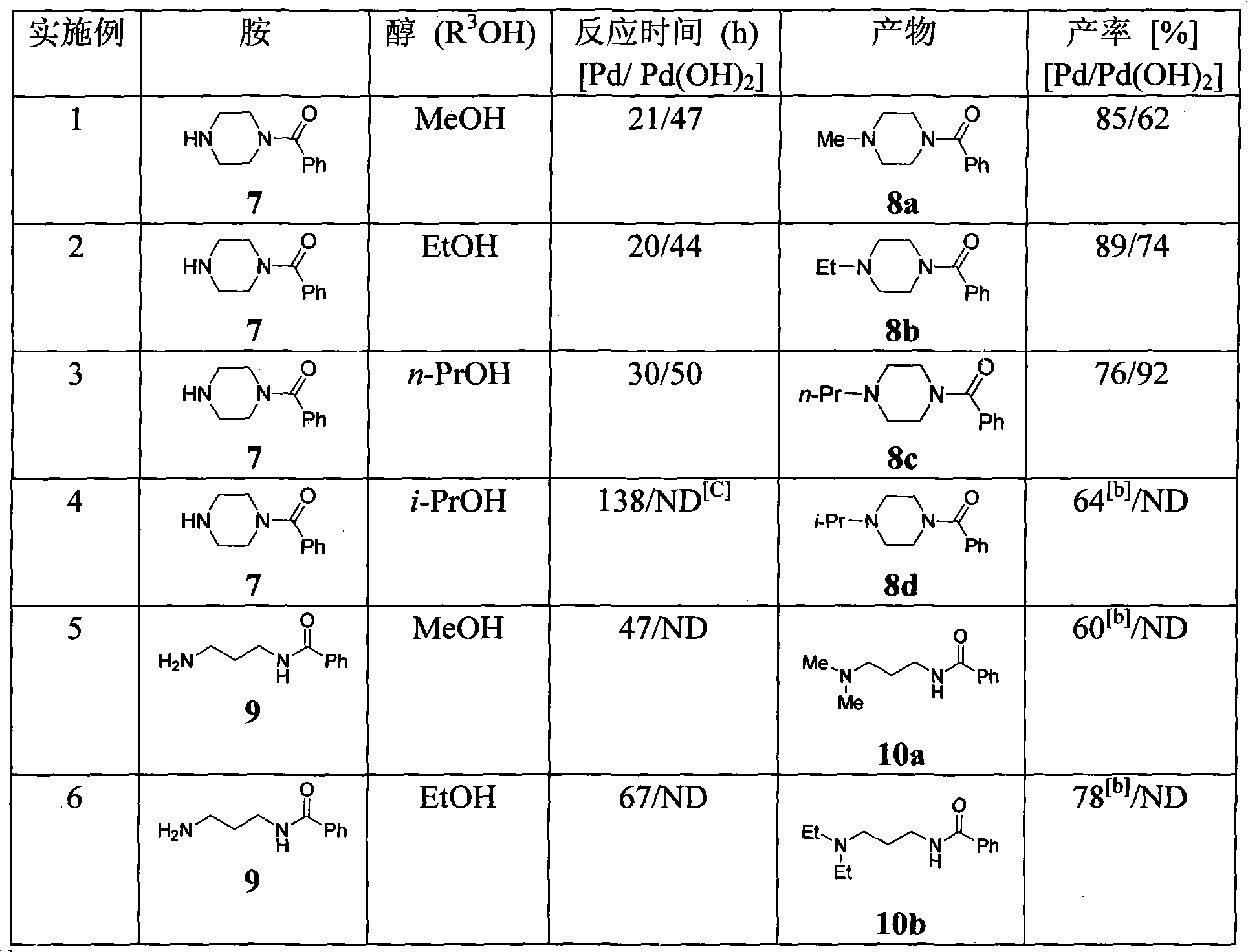
Method for alkylating amine and amino acid
An amino acid and alkylation technology, applied in the field of amines and amino acids, can solve the problems of insufficient mechanical stability, complex reaction system, limited validity period of catalysts, etc., and achieve the effect of good industrial application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Synthesis of 1-benzoyl-4-methylpiperazine (8a)
[0051]
[0052]Add 1-benzoylpiperazine 7 (0.1mmol, 19mg), methanol (5mL), 10% Pd / C (100mg) to the reaction flask, pump out the air in the flask under reduced pressure, inject hydrogen, and thin layer Chromatographic monitoring, 21h after the completion of the reaction. The catalyst was removed by vacuum filtration with a water pump, and then the filter cake was washed with 5 mL of methanol, and finally the filtrate was concentrated under reduced pressure to obtain 8a (17.3 mg, 85%) as a yellow oily liquid. In the same way, 10% Pd / C (100mg) was replaced by 20% Pd (OH) 2 / C (80mg), reacted for 47h to give 8a (12.6mg, 62%). IR(film)v max : 2936, 2850, 2786, 1632, 1424, 1296, 1271, 1168, 1141, 1128, 1019, 1004cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ: 2.31(s, 3H), 2.35(br s, 2H), 2.48(br s, 2H), 3.44(br s, 2H), 3.79(br s, 2H), 7.28-7.42(s, 5H) ; 13 C NMR (100MHz, CDCl 3 )δ: 42.0, 46.0, 47.5, 55.0 (2C), 127.0, 1...
Embodiment 2
[0053] Example 2 Synthesis of 1-benzoyl-4-ethylpiperazine (8b)
[0054]
[0055] Add 1-benzoylpiperazine 7 (0.1mmol, 19mg), ethanol (5mL), 10% Pd / C (100mg) to the reaction flask, pump out the air in the flask under reduced pressure, inject hydrogen, and thin layer Chromatographic monitoring, 20h after the completion of the reaction. The catalyst was removed by filtration with a water pump under reduced pressure, then the filter cake was washed with 5 mL of ethanol, and finally the filtrate was concentrated under reduced pressure to obtain 8b (19.4 mg, 89%) as a yellow oily liquid. In the same way, 10% Pd / C (100mg) was replaced by 20% Pd (OH) 2 / C (80 mg), reacted for 44 h to give 8b (16.1 mg, 74%). IR(film)v max : 2970, 2924, 2805, 1632, 1577, 1427, 1290, 1260, 1165, 1119, 1013, cm -1 ; 1 H NMR (400MHz, CDCl 3 )δ: 1.08(t, J=7.2Hz, 3H), 2.38(br s, 2H), 2.43(q, J=7.2Hz, 2H), 2.50(br s, 2H), 3.43(br s, 2H) , 3.79(br s, 2H), 7.36-7.40(s, 5H); 13 C NMR (100MHz, CDCl 3 )...
Embodiment 3
[0056] Example 3 Synthesis of 1-benzoyl-4-propylpiperazine (8c)
[0057]
[0058] Add 1-benzoylpiperazine 7 (0.1 mmol, 19 mg), n-propanol (5 mL), 10% Pd / C (100 mg) into the reaction flask, remove the air in the flask under reduced pressure, and inject hydrogen into the reaction flask. TLC monitoring, 30h after the completion of the reaction. The catalyst was removed by vacuum filtration with a water pump, then the filter cake was washed with 5 mL of n-propanol, and finally the filtrate was concentrated under reduced pressure to obtain 8c (17.6 mg, 76%) as a yellow oily liquid. In the same way, 10% Pd / C (100mg) was replaced by 20% Pd (OH) 2 / C (80 mg), reacted for 50 h to give 8c (21.3 mg, 92%). IR(film)v max 2964, 2921, 2866, 2805, 2765, 1632, 1427, 1372, 1293, 1278, 1159, 1015, 1001cm -1 ; 1 H NMR (500MHz, CDCl 3 )δ: 0.91(t, J=7.4Hz, 3H), 1.51(tqapparent sextet, J=7.7, 7.4Hz, 2H), 2.33(t, J=7.7Hz, 2H), 2.38(br s, 2H), 2.52(br s, 2H), 3.44(br s, 2H), 3.50(br s, 2H,)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com