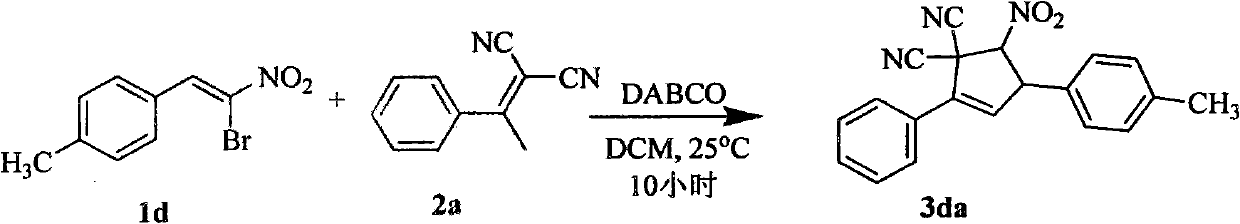
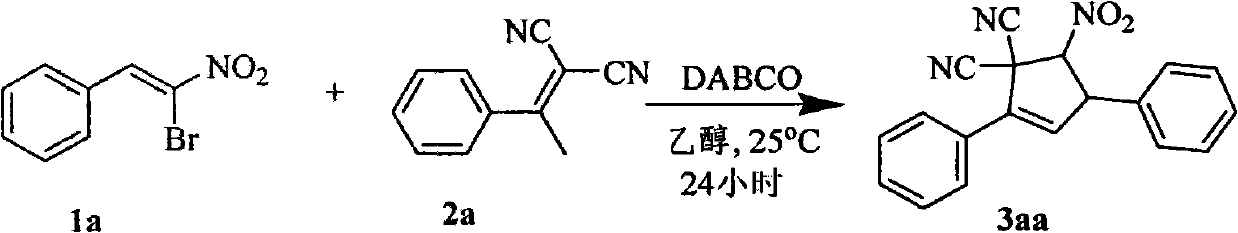
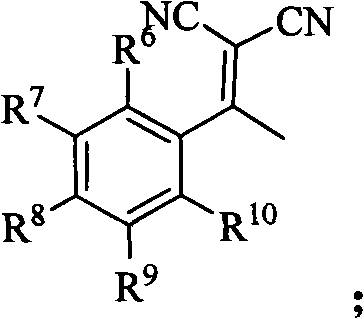
Synthetic method of 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and derivative thereof
A synthesis method and diphenyl technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitriles, etc., can solve the problems of no literature reports, serious environmental pollution, and inability to meet industrial production, and achieve applicable The effect of wide range, high yield and flexible reaction time
Inactive Publication Date: 2010-10-06
ZHEJIANG NORMAL UNIVERSITY
View PDF0 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, existing synthetic methods all need to adopt toxic metals as catalysts, for example: a kind of reductive cyclization reaction (Synthetic Communications, 32(21), 3311-3317; 2002), and vanadium-catalyzed stereo dimerization of arylmethylene malononitriles in the presence of chlorine and zinc (T-etrahedron Letters, 41(44), 8517- 8521; 2000), the above two methods are catalyzed by toxic metals, which seriously pollute the environment and cannot meet the needs of industrial production
And about 5-nitro-2,4-diphenylcyclopent-2-ene-1,1-dinitrile (5-nitro-2,4-diphenylcyclopent-2-ene-1,1-dicarbonitrile) and its The study of derivatives has not been reported in the literature so far
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a synthetic method of 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and a derivative thereof, which comprises the following steps of: in the presence of a catalyst, adding substituted or non-substituted 1-bromine-1-nitryl-2-styrene and substituted or non-substituted 1,1-dinitrile-2-methyl-2-styrene into a reaction solvent for reaction, and post processing a reaction product to obtain the 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and the derivative thereof. The method has the advantages of flexible reaction time, higher yield, low price and easy obtainment of most solvents, environmental protection, simple operation, wide application range and the like and is suitable for industrialized production. The invention also discloses the 5-nitryl-2,4-diphenyl cyclopentyl-2-ene-1,1-dinitrile and the derivative thereof, which have the following structural formulae; and the compound has better antibacterial and insecticidal actions.
Description
technical field The invention relates to the field of synthesis of organic compounds, in particular to a synthesis method of 5-nitro-2,4-diphenylcyclopent-2-ene-1,1-dinitrile and derivatives thereof. Background technique Cyclopentene and its derivatives are an important class of organic compounds. As the structural units of some natural products and drugs, they have a wide range of biological and pharmacological activities. For example, many cyclopentene derivatives have physiological effects such as antibacterial, fish poisoning, insecticide, weed control, antiallergic and anticancer. Therefore, the synthesis of cyclopentene and its derivatives has broad application prospects. At present, the method for the synthesis of cyclopentene derivatives commonly used is as follows reaction formula (a): However, existing synthetic methods all need to adopt toxic metals as catalysts, for example: a kind of reductive cyclization reaction (Synthetic Communications, 32(21), 3311-3...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07C253/30C07C255/46
Inventor 谢建武尚昊罗玲玲
Owner ZHEJIANG NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com