Combination therapy related to serotonin dual action compounds
A composition, hydroxytryptophan technology, applied in the direction of drug combination, organic chemistry, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
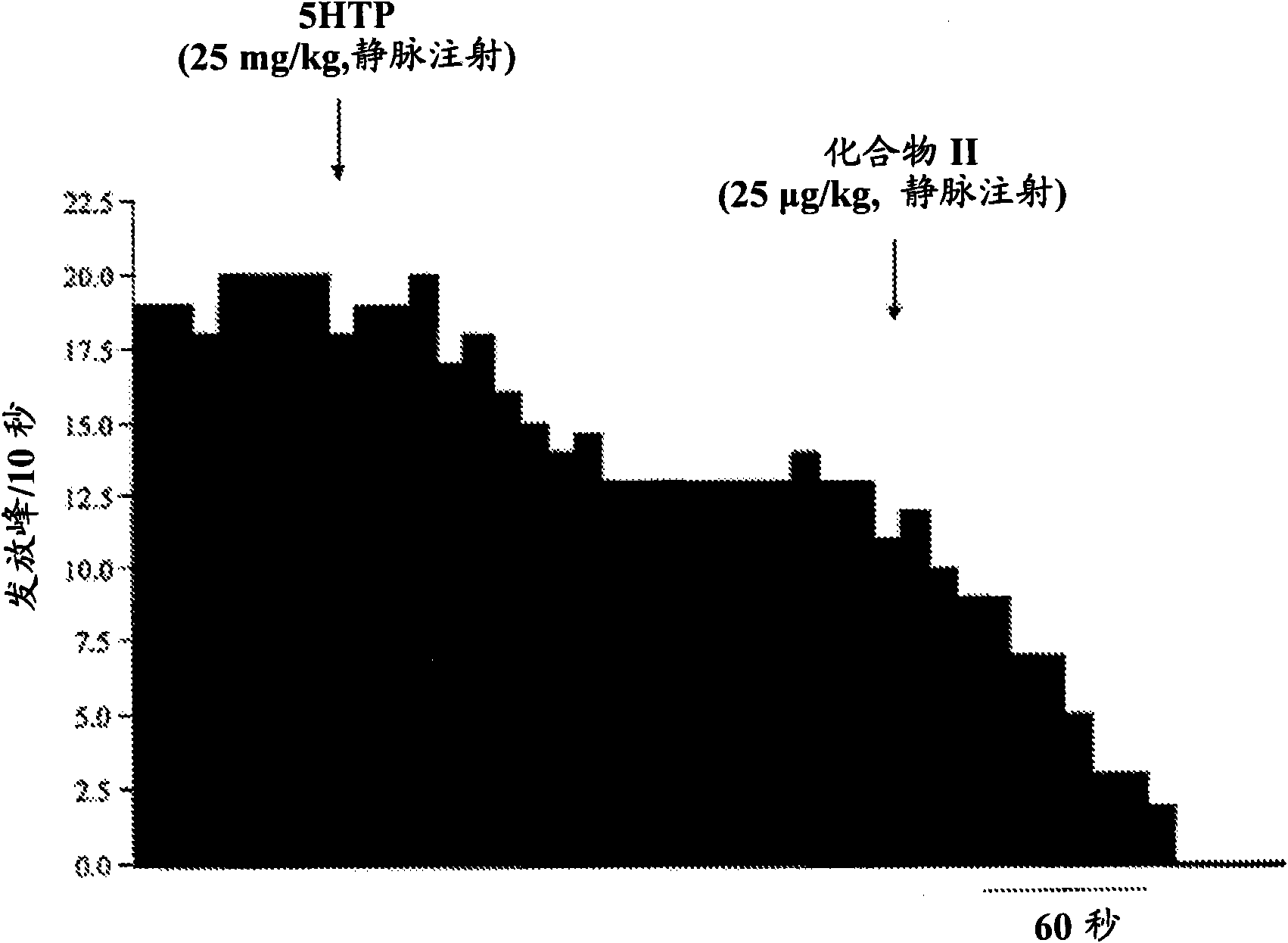
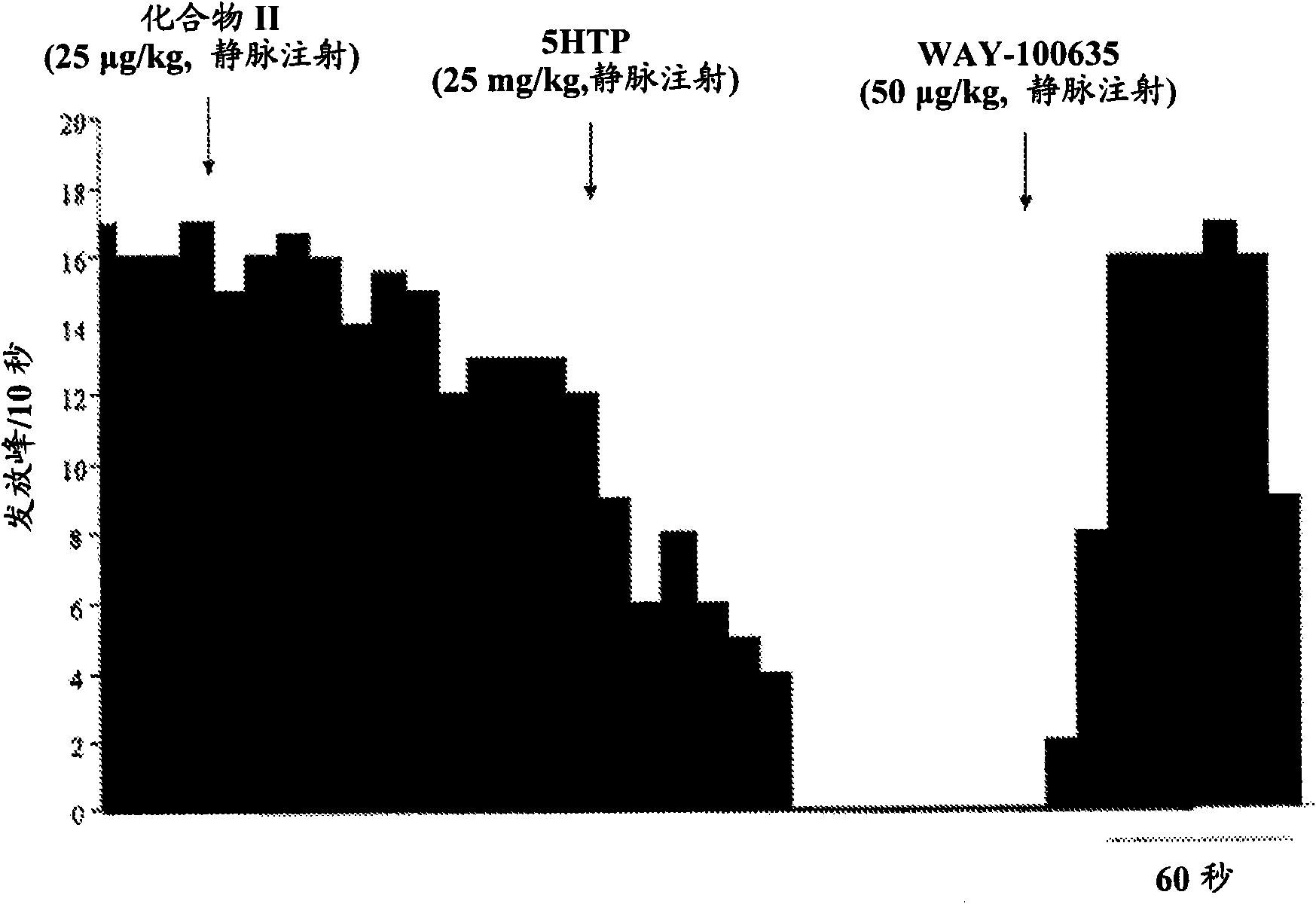
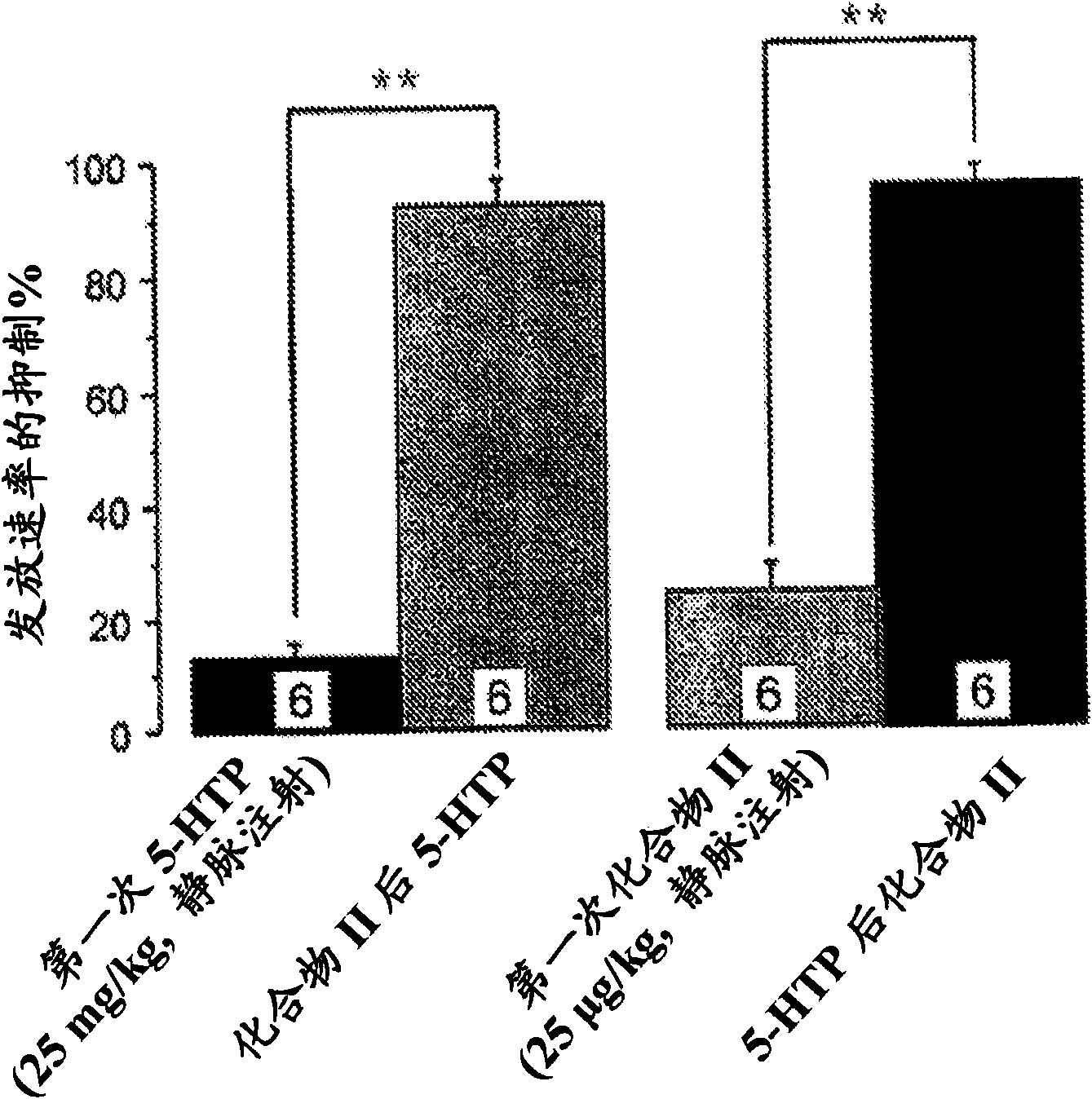
[0015] The present invention relates to serotonin reuptake inhibitors such as 2-(6-fluoro-1H-indol-3-ylsulfanyl)-benzyl]- Pharmaceutical compositions of methyl-amines.
[0016] As used herein, "serotonin reuptake inhibitor" or "SRI" refers to a drug that inhibits serotonin reabsorption in the central nervous system (CNS) mainly or in part by binding to the primary ligand binding site of the serotonin transporter. Compounds that exert their therapeutic effects.
[0017] As used herein, "allosteric modulator" shall refer to an SRI that binds to the allosteric site of the serotonin transporter (SERT). Such compounds are also known as allosteric serotonin reuptake inhibitors (ASRIs).
[0018] In one embodiment of the invention, the allosteric modulator binds to one or more allosteric sites of SERT. In another embodiment, the allosteric modulator binds to the allosteric site of SERT capable of binding 2-(6-fluoro-1H-indol-3-ylsulfanyl)-benzyl]-methyl-amine .
[0019] 5-Hydroxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com