Pyrimidine-ring-contained palladium metal ligand and preparation method thereof
A technology of metal ligands and pyrimidinyl rings, which is applied in the field of organic synthesis, can solve the problems of strong coordination ability and multi-dentate ligand coordination sites, and achieve the effects of stable structure, low requirements for reaction conditions, and convenient storage and transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
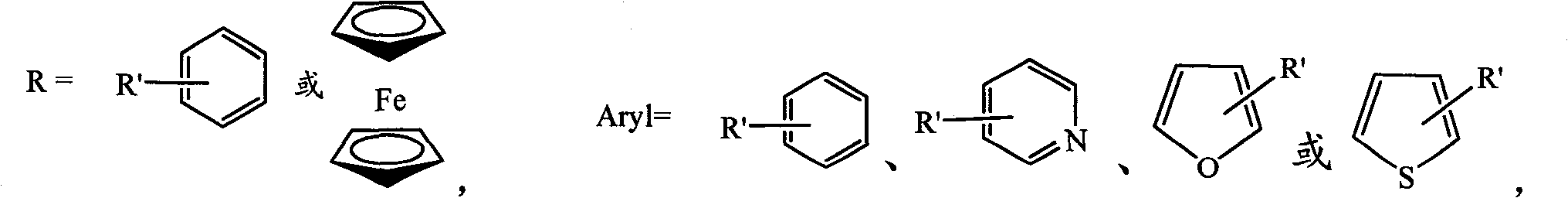
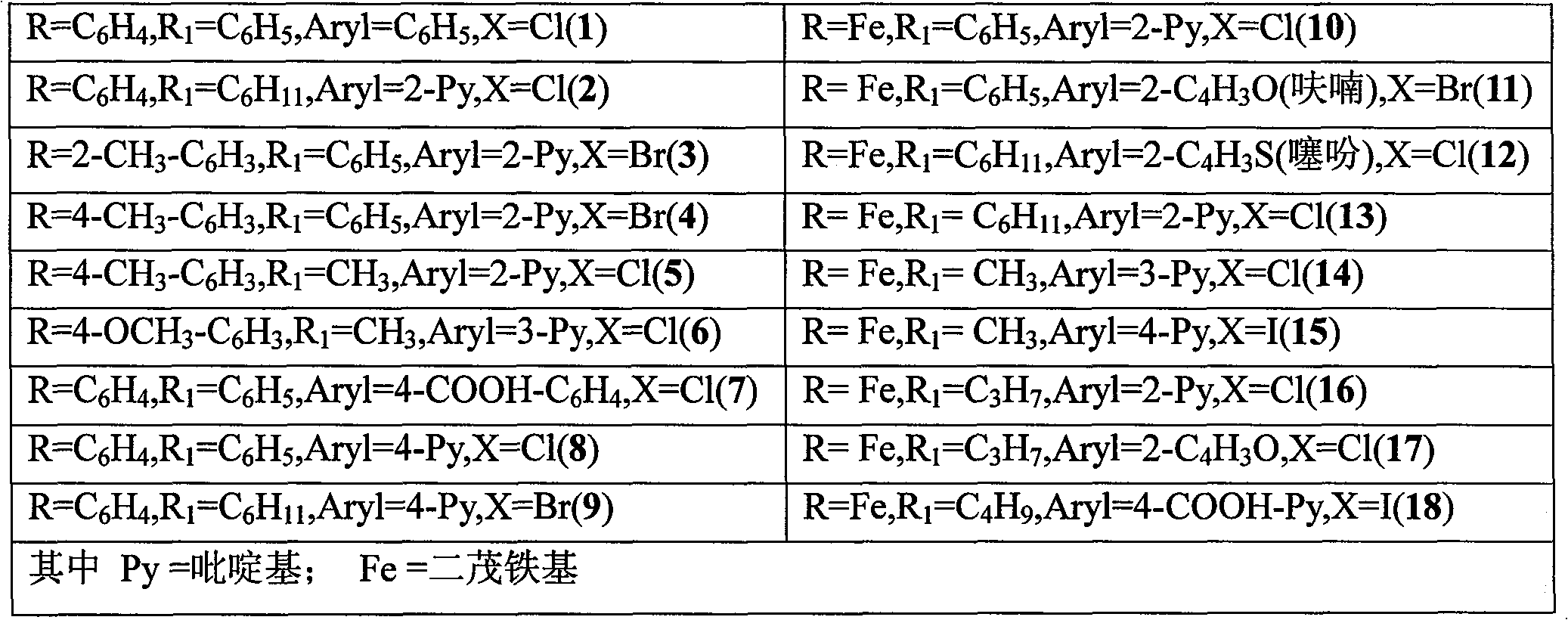
[0023] Containing pyrimidinyl cyclopalladium metal ligands, the general formula is:
[0024] Where the dashed part is R:
[0025]
Embodiment 2
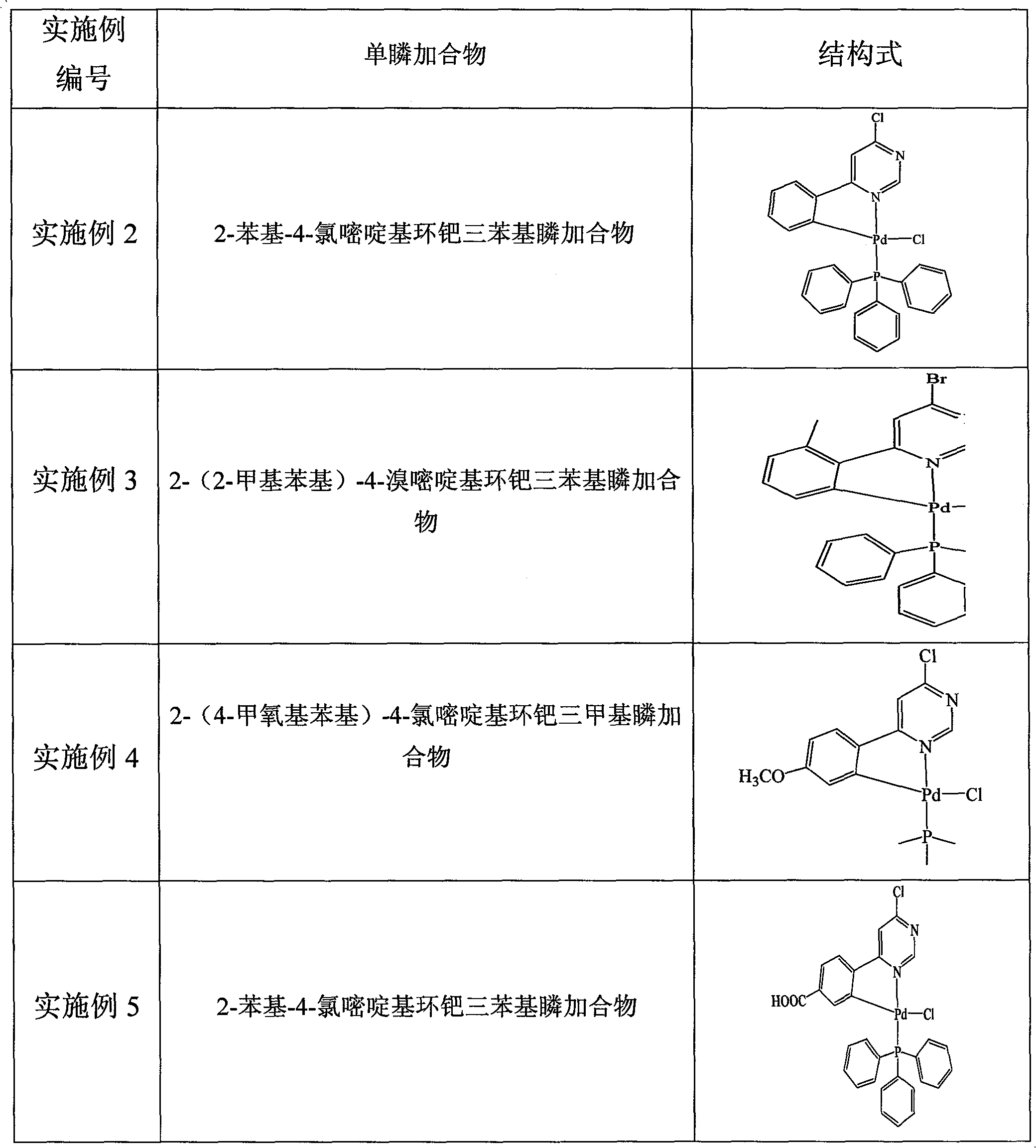
[0027] 2, the preparation of 4-diphenylpyrimidinyl cyclopalladium metal ligand (1): under the protection of an inert gas (such as high-purity nitrogen), the Schlek reaction tube (a kind of commonly used during anhydrous and anaerobic operation) of 10ml Glass instrument) add 0.5mmol 2-phenyl-4-chloropyrimidinyl cyclopalladium triphenylsulfone adduct, 0.6mmol phenylboronic acid, 1mmol potassium carbonate, replace the reaction tube with nitrogen for 3 times, and Under the continuous protection of nitrogen, 5ml of toluene solvent was added with a syringe, and then heated to 110°C with an oil bath under magnetic stirring, and the reaction was refluxed for 12 hours.
[0028] After the reaction, the oil bath was removed, and the water bath was lowered to room temperature; 3ml of water was added to the reaction solution, and then extracted three times with 5ml of dichloromethane, the organic phases were combined and washed with anhydrous MgSO 4 Dry for 30 minutes and filter; the filtr...
Embodiment 3
[0030] Preparation of 2-(2-methylphenyl)-4-(2-pyridyl)pyrimidinyl cyclopalladium metal ligand (3): under nitrogen protection, add 0.5mmol 2-(2 -Methylphenyl)-4-bromopyrimidinyl cyclopalladium triphenylsulfone adduct, 0.5mmol of 2-pyridylboronic acid, 0.5mmol of sodium carbonate; replace the reaction tube with nitrogen for 3 times, and in slight positive pressure nitrogen Add 5ml of dioxane solvent with a syringe under the continuous protection of ; heat to 90°C with an oil bath under magnetic stirring, and reflux the reaction for 8 hours.
[0031] After the reaction, the oil bath was removed, and the water bath was lowered to room temperature; 3ml of water was added to the reaction solution, and then extracted three times with 5ml of dichloromethane, the organic phases were combined and washed with anhydrous MgSO 4 Dry for 30 minutes and filter; the filtrate is concentrated by a rotary evaporator, and the concentrated residue is separated by silica gel thin-layer chromatograph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com