Combination therapy with antibody-drug conjugates
A technology for coupling compounds and antibodies, applied in the field of combined therapy with antibody-drug conjugates, can solve the unmet medical needs of HL patients and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
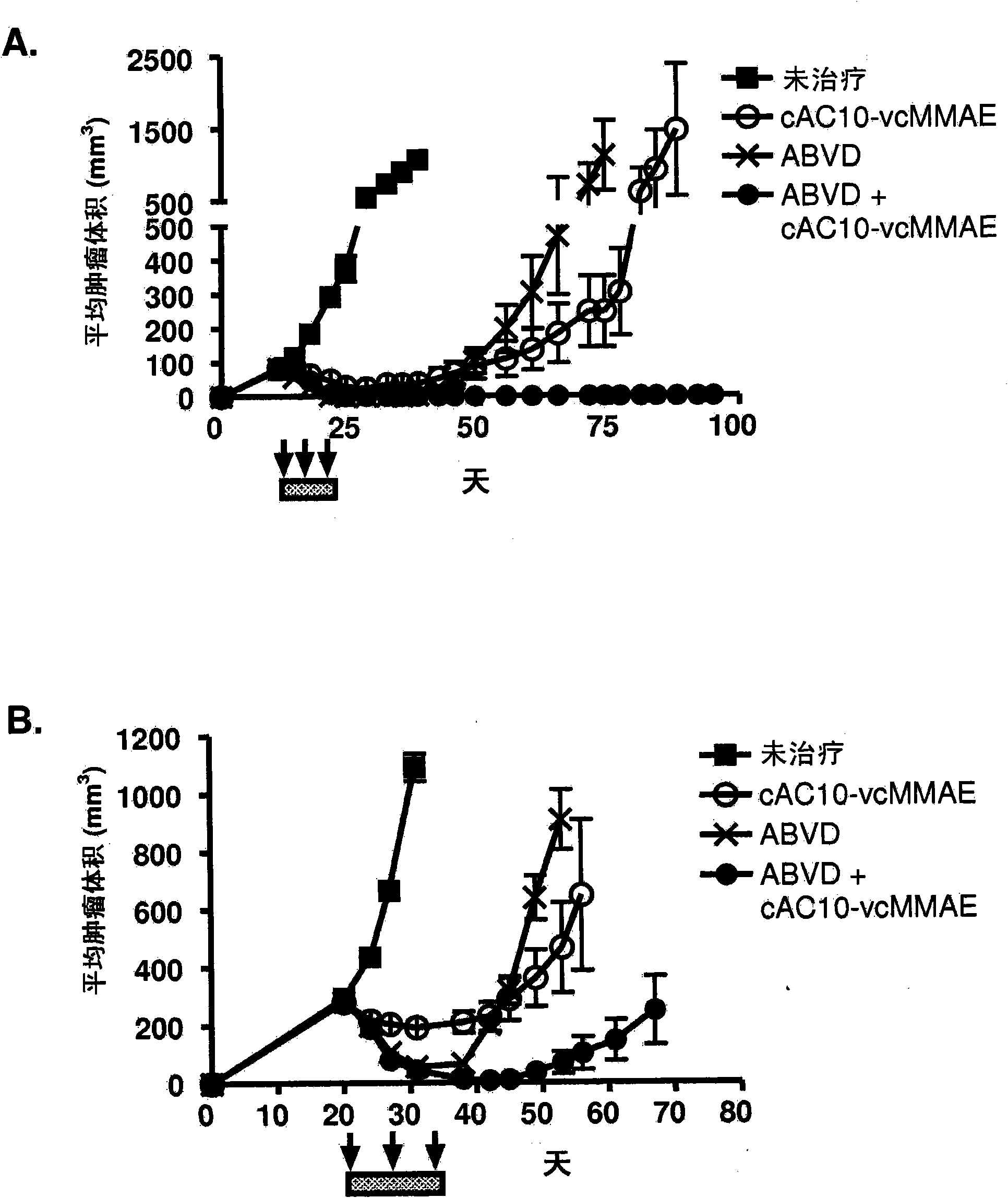
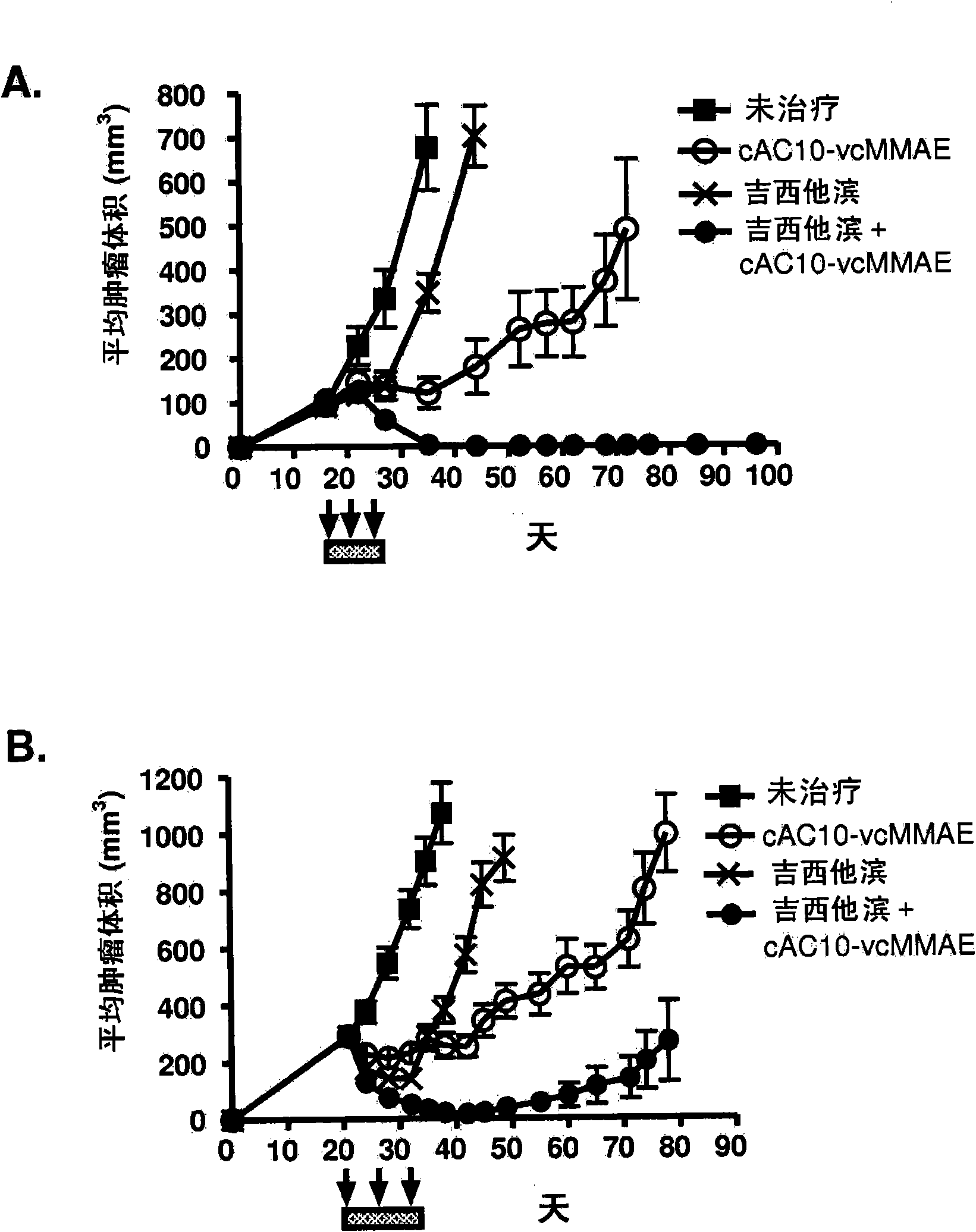
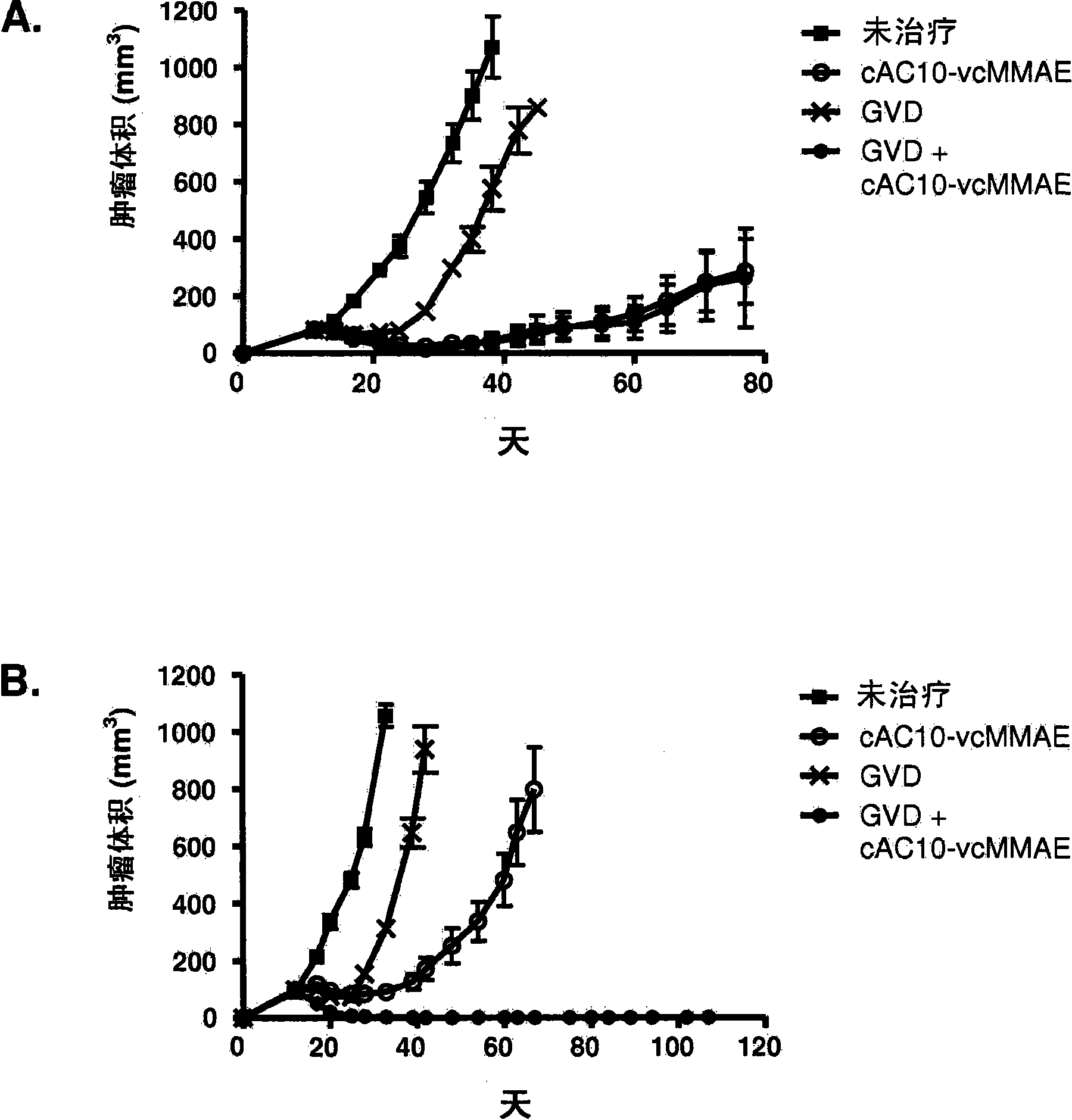
[0480] Example 1 : Antibody-drug conjugate cAC10-vcMMAE (cAC10-MC-vc-PAB-MMAE) in combination with chemotherapy regimens for the treatment of HL
[0481]The effect of combined cAC10-vcMMAE therapy with gemcitabine or ABVD or other chemotherapy regimens was studied in the L540cy tumor model (Francisco et al., Blood 2003; 102:1458-146). To determine the maximum tolerated dose (MTD) of ABVD and gemcitabine, body weights of SCID mice treated with increasing amounts of drug were assessed daily. MTD was assessed by ≥20% weight loss or other signs of morbidity throughout the treatment period and subsequent 2-week recovery period. The time to tumor quadrupling or tripling was chosen as the time to endpoint (TTE), and nonlinear regression analysis was performed on individual tumor growth data sets for each experimental animal in exponential growth phase to determine this endpoint. Time to tumor quadrupling was calculated based on tumor volume at the start of treatment. The TTE valu...
Embodiment 2
[0505] Example 2: Antibody-drug conjugate cAC10-mcMMAF combined with gemcitabine for the treatment of HL
[0506] The effect of cAC10-mcMMAF treatment in combination with gemcitabine was studied in the same manner as the experiments used for cAC10-vcMMAE. Choose 1 mg / kg, q4dx3 regimen for cAC10-mcMMAF. On Day 51 of the trial, 9 / 10 sustained responses were observed in the combination arm compared with 0 / 10 in the cAC10-mcMMAF monotherapy arm and 0 / 10 in the gemcitabine single arm arm. 10 sustained responses. The experiment is on day 51 and has not yet been completed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com