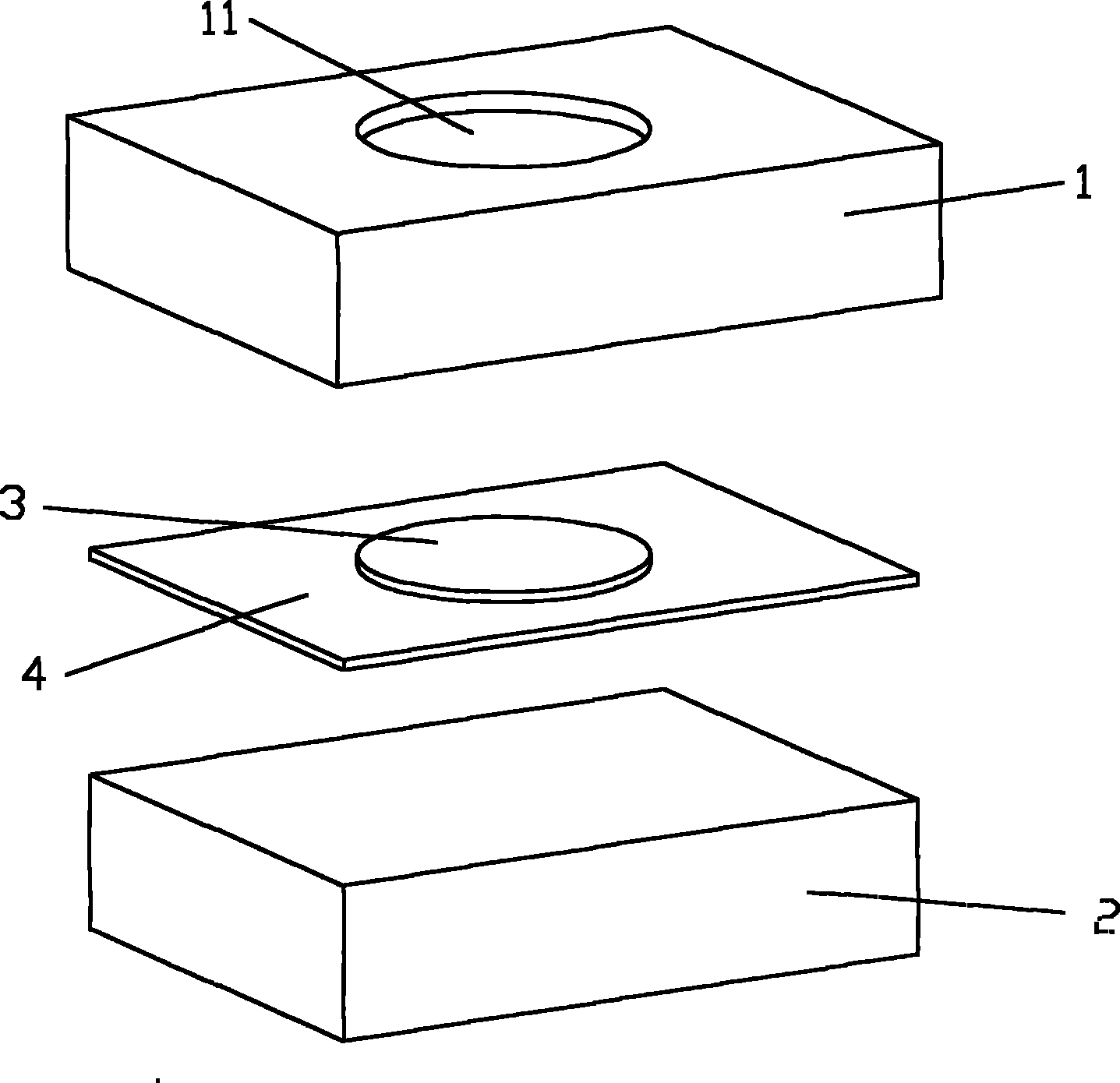
Composition for detecting rheumatoid arthritis immune antibody by dot immuno-gold filtration assay
A technology of immune filtration and immune antibody, applied in the direction of anti-animal/human immunoglobulin, measuring device, animal/human peptide, etc., can solve the problem of BiP rheumatoid arthritis without data support, waiting for further independent clinical research , the impact of detection sensitivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] The synthesis of embodiment 1 polypeptide I
[0086] Polypeptides are synthesized by solid-phase synthesis technology using Fmoc chemical method. For the specific steps of this method, see Eur.J.Immunol.1994, 24, 3188-3193; J.Org.Chem.1972, 37, 3404-3409; Huang Weide, Chen Changqing Polypeptide Synthesis, Beijing: Science Press, 1985.
[0087] The formation method and steps of disulfide bonds can be found in the literature: Huang Weide, Chen Changqing Peptide Synthesis, Beijing: Science Press, 1985, p85; Michael W.Pennington Peptide Synthesis Protocols (Methods in Molecular Biology), Humana Press, 1994, p91-169 .
[0088] Through the above steps, the specific sequence of the synthetic polypeptide is:
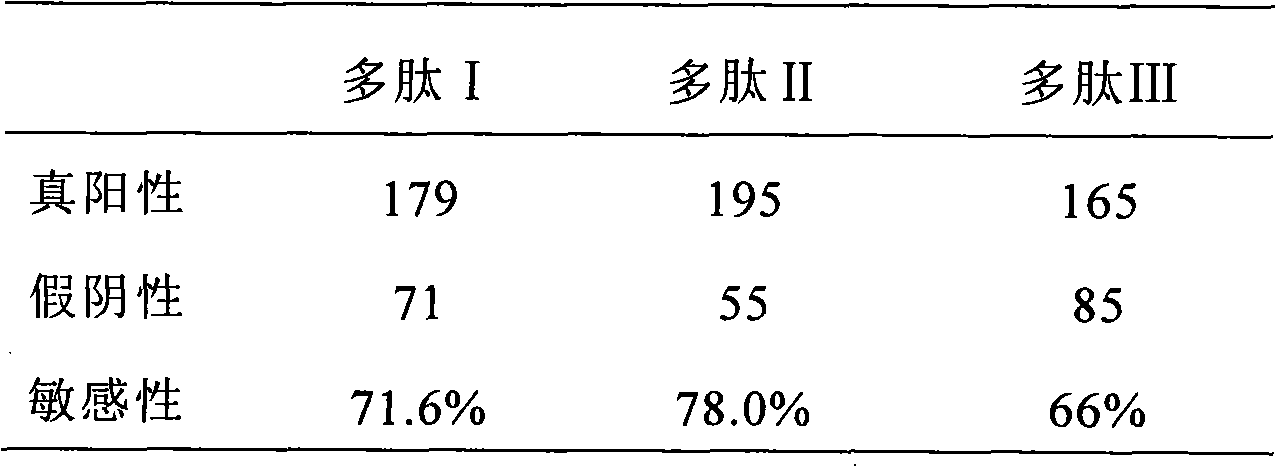
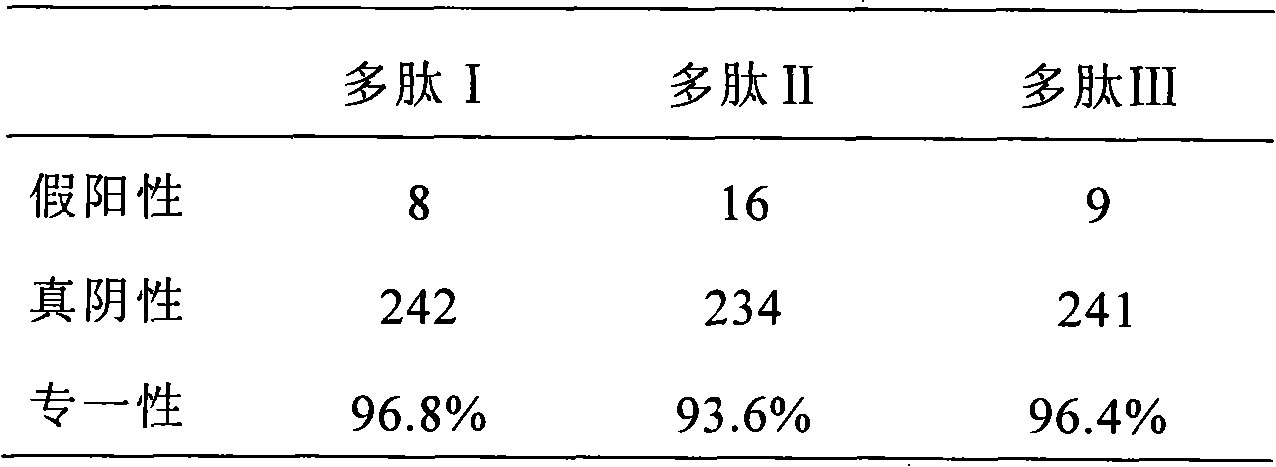
[0089] Polypeptide I: His-Gln-Cys-Ala-Arg-Phe-Gln-Met-Arg-His-Cit-Arg-Leu-Ile-Arg-Cys-Gly, on which the third and sixteenth positions of Cys pass through the sulfhydryl group Form a disulfide bond, and make the polypeptide form a ring structure, and can simulate the β-...
Embodiment 2
[0090] The synthesis of embodiment 2 polypeptide II
[0091] Polypeptide II was synthesized according to the method disclosed in Example 1: His-Gln-Cys-Ala-Arg-Phe-Gln-Met-Cit-His-Cit-Arg-Leu-Ile-Arg-Cys-Gly, on which the third The Cys at the sixteenth position and the sixteenth position form a disulfide bond through the sulfhydryl group, and make the polypeptide form a ring structure, and can simulate the β-turn structure.
Embodiment 3
[0092] The synthesis of embodiment 3 polypeptide III
[0093] Polypeptide III was synthesized according to the method disclosed in Example 1: His-Gln-Cys-His-Gln-Phe-Arg-Phe-Cit-Gly-Cit-Ser-Arg-Ala-Ala-Cys-Gly, on which the third The Cys at the sixteenth position and the sixteenth position form a disulfide bond through the sulfhydryl group, and make the polypeptide form a ring structure, and can simulate the β-turn structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com