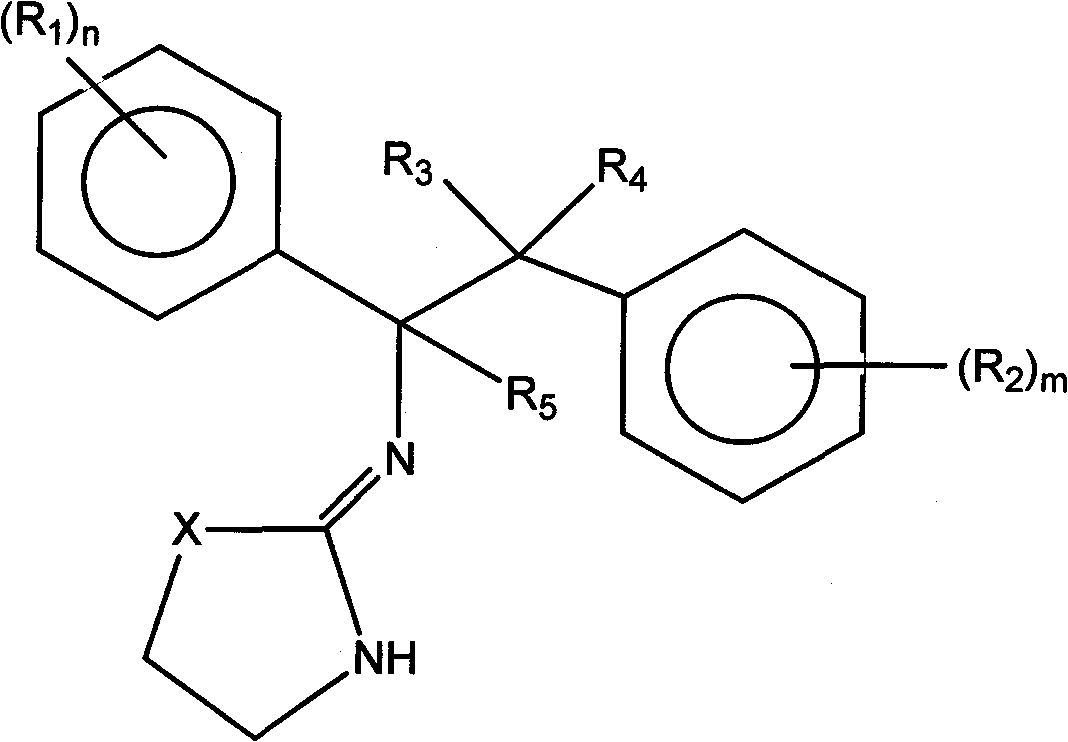
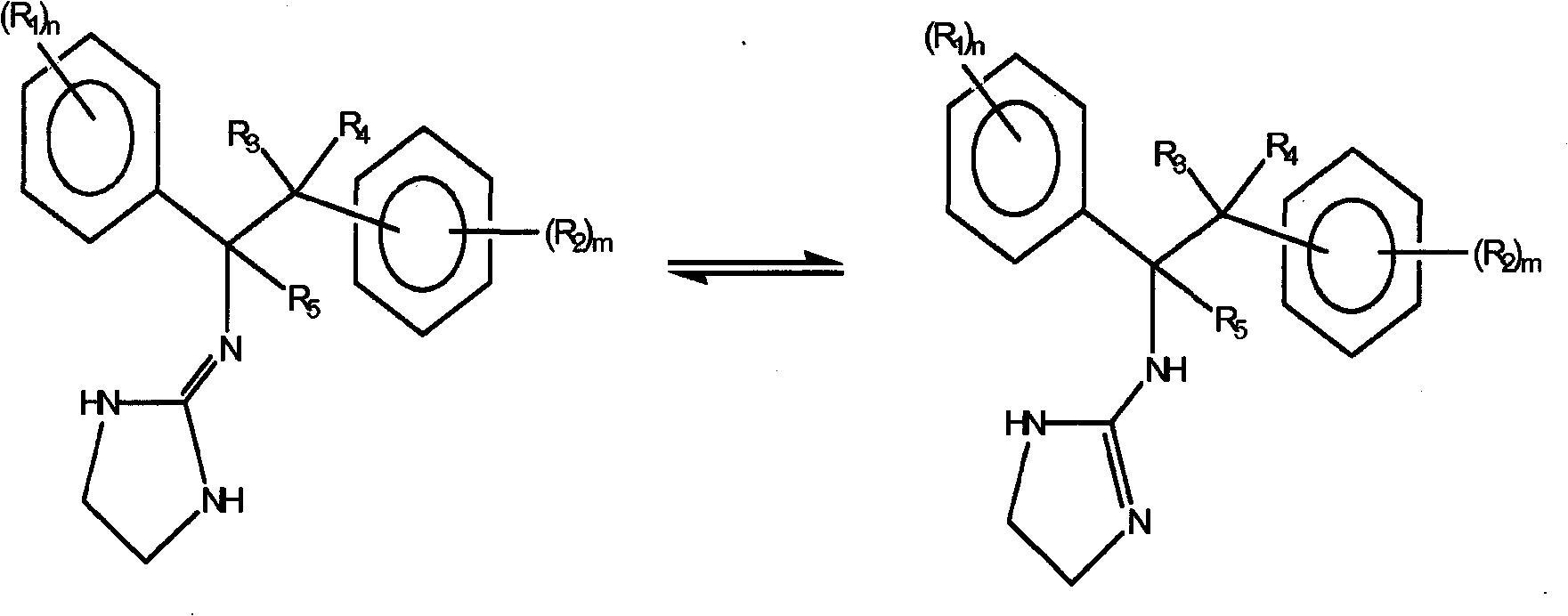
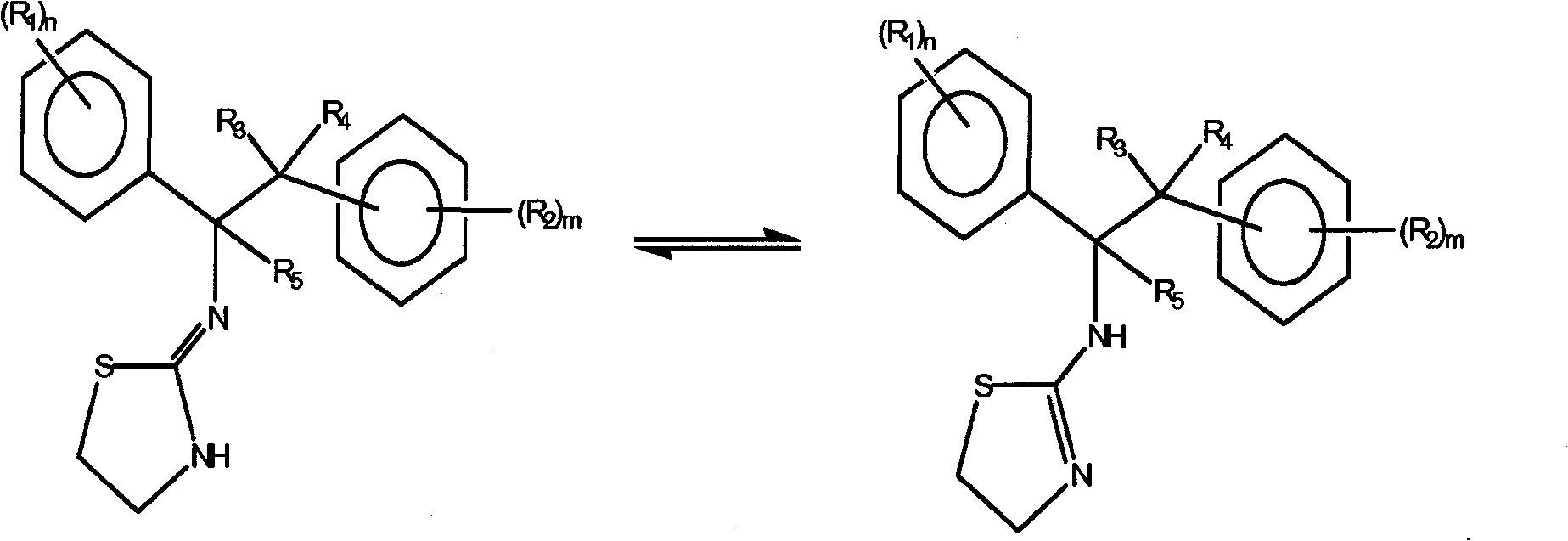
Selective subtype alpha 2 adrenergic agents and methods for use thereof
A technology of drugs and compositions, applied in drug combinations, pharmaceutical formulations, antipyretics, etc., can solve problems such as adverse side effects, hypotension and sedative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0107] General synthesis of amine precursors
[0108]
[0109] A. 3-Chloro-2-methylbenzaldehyde
[0110] To a solution of 3-chloro-2-methylbenzonitrile (5 g, 33 mmol) in dichloromethane (150 mL) was added DiBAL (1 M in dichloromethane, 41 mL) at -78°C. The reaction mixture was stirred at -78°C for 2 hours and then quenched with methanol. The mixture was warmed to 0 °C, then HCl (10%) was added. The ice-water bath was removed, and the mixture was stirred at room temperature for 10 minutes. The two phases were separated and the aqueous phase was extracted with dichloromethane. The combined dichloromethane was washed with brine, dried over sodium sulfate and concentrated. 3-Chloro-2-methylbenzaldehyde (3.5 g, 69%) was obtained by column chromatography (5% ethyl acetate / n-hexane).
[0111] 1 H NMR (300MHz, CDCl 3 )δ2.64(s, 3H), 7.21-7.26(m, 1H), 7.50-7.53(m, 1H), 7.63-7.66(m, 1H), 10.20(s, 1H)
[0112] B. 1-(3-Chloro-2-methylphenyl)-2-phenylethylamine
[0113] To a sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com