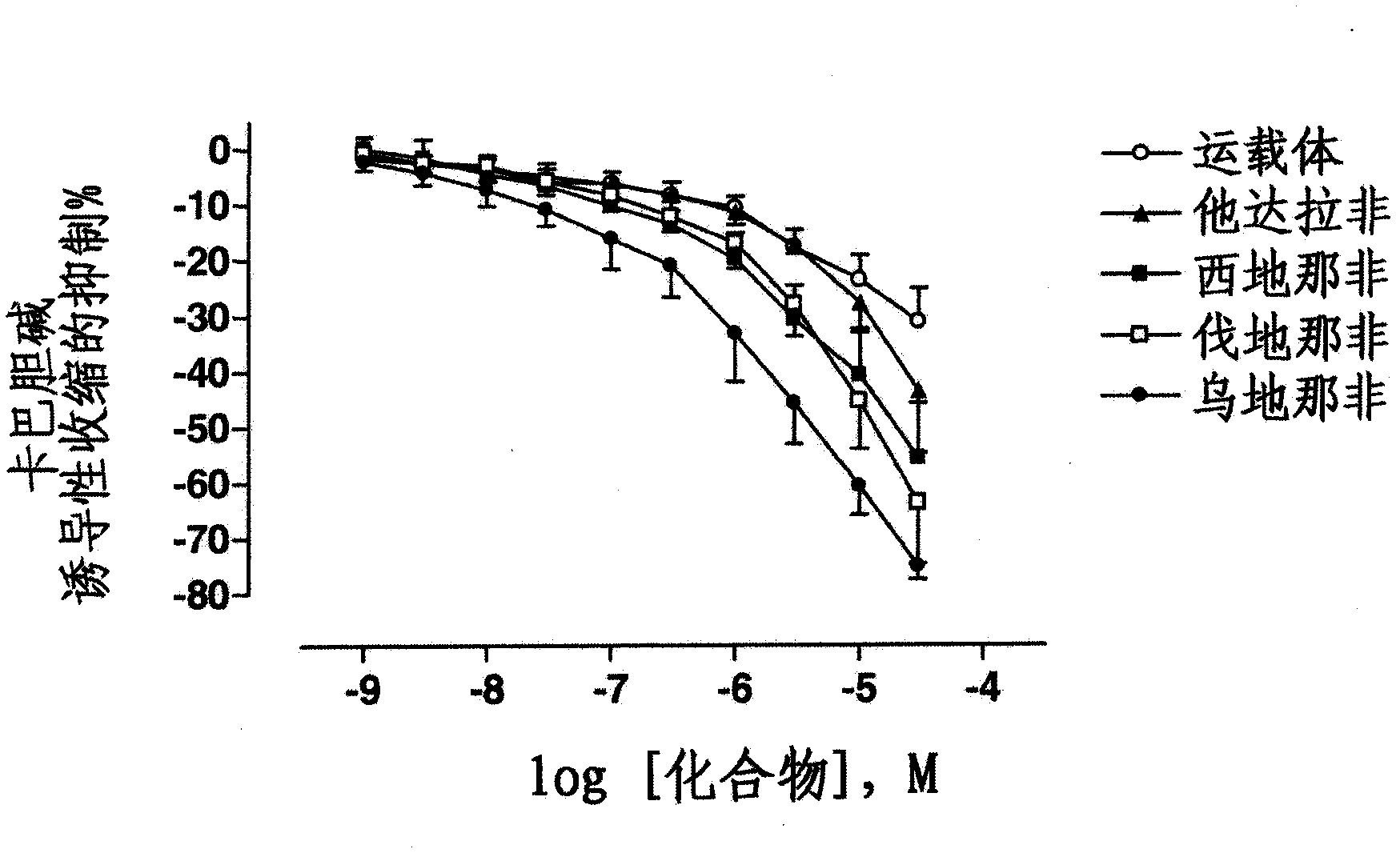
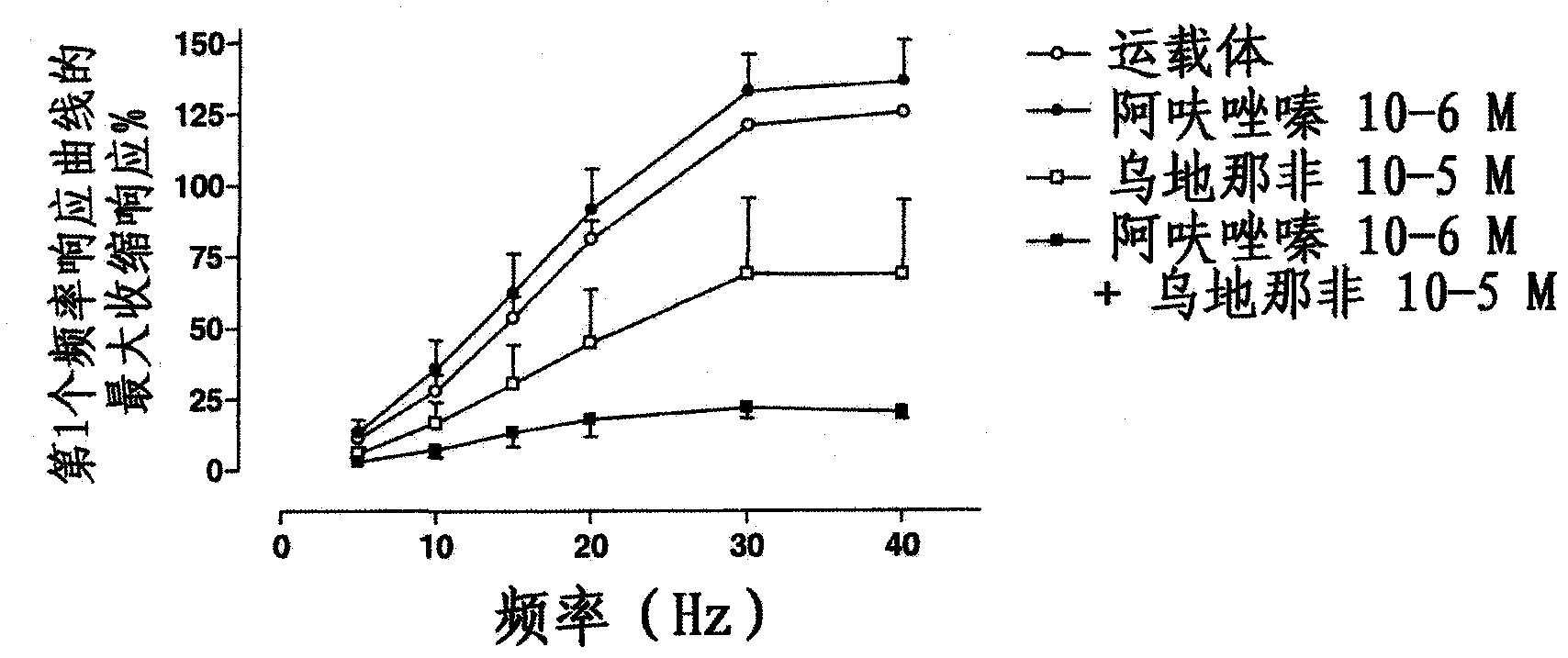
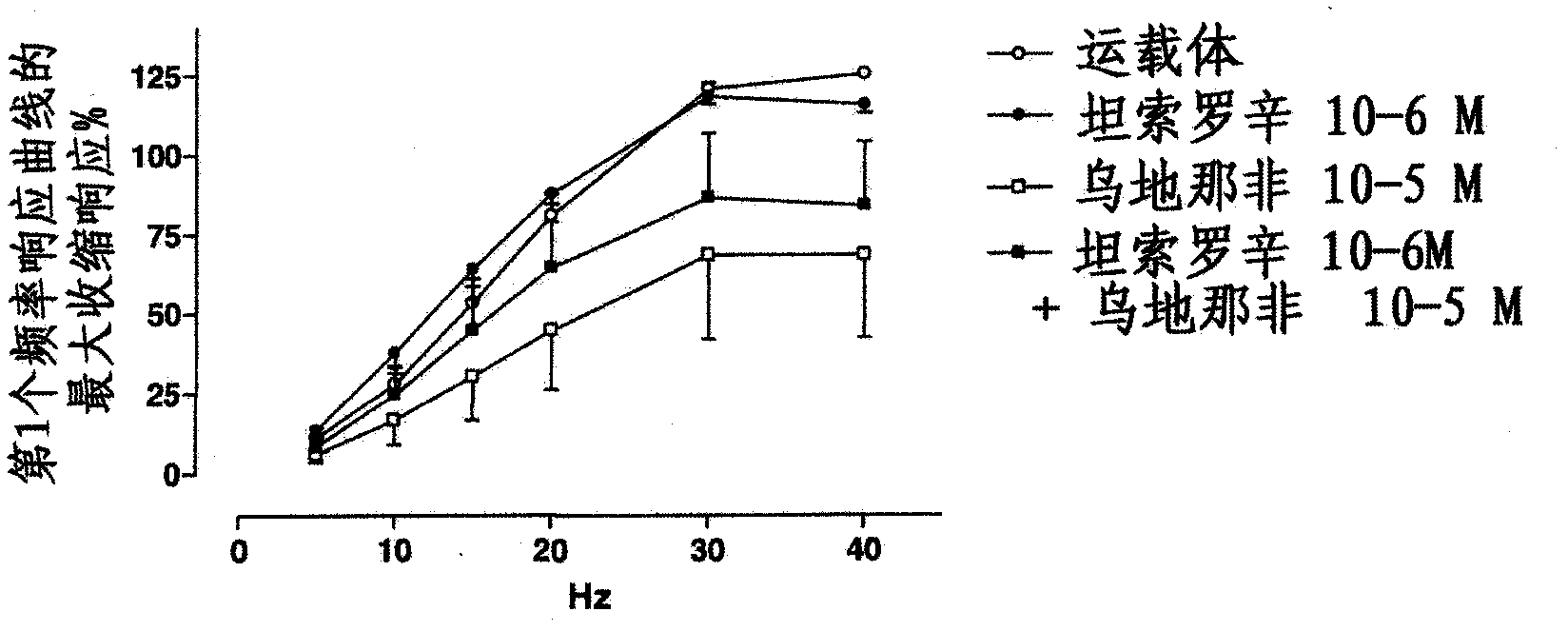
Use of a combination of udenafil and alfuzosin or oxybutynin for the treatment of overactive bladder.
A technology of alfuzosin and udenafil, applied in the field of drugs for the treatment of overactive bladder, which can solve the unmet and growing huge demand for effective and acceptable medical treatment of overactive bladder, accompanied by side effects, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0082] The following examples are provided to illustrate the invention but are not intended to limit its scope.
[0083] Materials and methods
[0084] human bladder strip
[0085] The experiments, collection and use of any tissue or other samples were carried out in accordance with the research plan, all relevant laws, regulations and industry rules (including having obtained written informed consent from patients).
[0086] Bladders were obtained from donors who underwent cystectomy for invasive bladder cancer and had no known bladder dysfunction according to their medical charts. In accordance with French legislation, human tissue samples were obtained with the informed consent of the patients and after serological testing for hepatitis and HIV.
[0087] Immediately after surgery, bladder samples were transferred from the operating room to the pathologist's laboratory, who selected a piece of normal bladder dome (ie, no macroscopic tumor tissue) for experimental purposes....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com