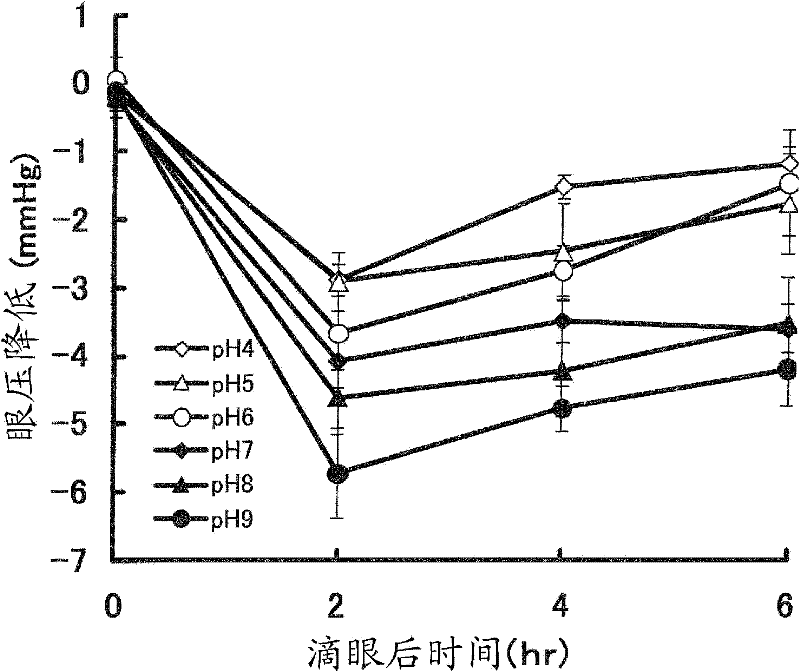
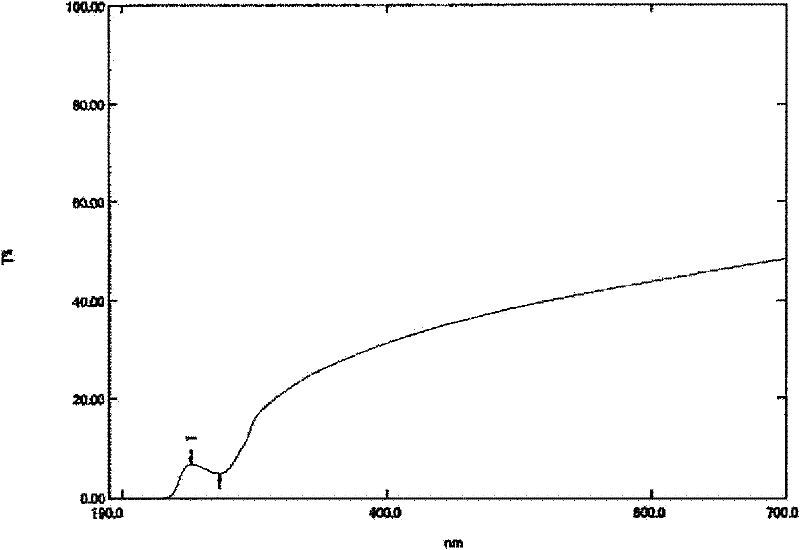
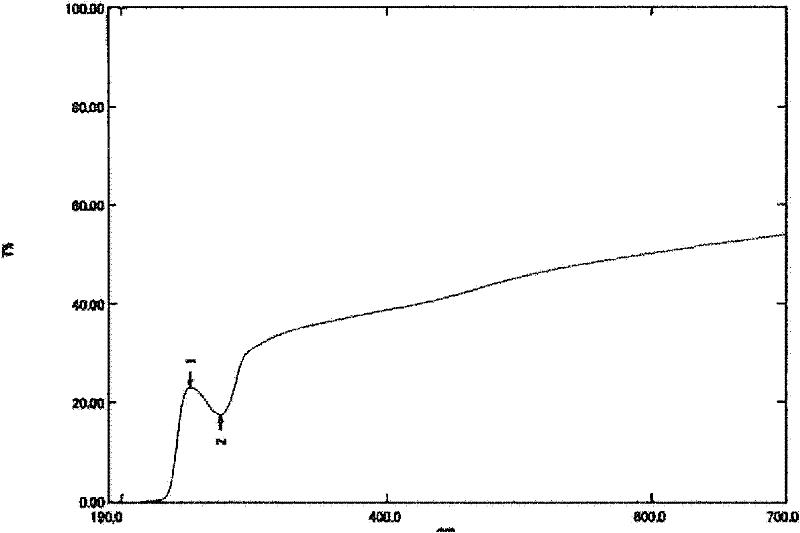
Stable aqueous solution composition containing sulfonamide compound
An aqueous solution and composition technology, applied in the field of aqueous solution composition, can solve problems such as poor patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0081] [Example 1] Preparation of (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-(methylamino)pyrrolidine monohydrochloride
[0082] According to the method described in International Patent Publication No. WO2007 / 026664 pamphlet, (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-(methyl amino)pyrrolidine, which was dissolved in water (52 mL). The solution was vigorously stirred, and a 2 mol / L sodium hydroxide aqueous solution (4.13 mL, manufactured by Wako Pure Chemical Industries, Ltd.) was slowly added dropwise under ice-cooling. After the resulting suspension was further stirred at room temperature for 1 hour, dichloromethane (30 mL) was added, and the organic layer was separated. The aqueous layer was further extracted with dichloromethane (30 mL), and the combined organic layers were washed with water (50 mL), and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, and ethyl acetate (10 mL) and n-hexane (20 mL) were added to the residue. T...
example 2
[0085] [Example 2] Preparation of (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-(methylamino)pyrrolidine monohydrochloride aqueous solution not containing citric acid or its salt
[0086] (1) Physiological saline solution
[0087] Make a solvent by dissolving 18g of sodium chloride in 2L of distilled water. Add 60 mL of solvent to dissolve 12 mg of (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-(methylamino)pyrrolidine monohydrochloride, and measure the pH.
[0088] (2) 10mM phosphate buffer (pH6) solution
[0089] Dissolve 0.78 g of sodium dihydrogen phosphate dihydrate in 500 mL of the solvent of Example 2 (1). A solution obtained by dissolving 1.432 g of disodium hydrogenphosphate dodecahydrate in 400 mL of the solvent of Example 2 (1) was added to 50 mL of this liquid to adjust the pH to 6.0, and this was prepared as a 10 mM phosphate buffer (pH 6) solvent. Add 60mL of 10mM phosphate buffer (pH6) solvent to 12mg of (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-(methylamino)pyrrolidi...
example 3
[0152] [Example 3] Preparation of (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-aminopyrrolidine monohydrochloride
[0153] Hydrochloride (5.500 g) of (S)-1-(4-chloro-5-isoquinolinesulfonyl)-3-aminopyrrolidine obtained by the method described in International Patent Publication No. WO2007 / 026664 pamphlet Dissolve in water (50mL). The solution was vigorously stirred, and a 1 mol / L sodium hydroxide aqueous solution (29.32 mL, manufactured by Wako Pure Chemical Industries, Ltd.) was slowly added dropwise under ice-cooling. Ethyl acetate (300 mL) and water (20 mL) were added, and the organic layer was distilled off. The aqueous layer was further extracted with dichloromethane, and the combined organic layers were washed with water and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, and ethyl acetate (35 mL) and n-hexane (105 mL) were added to the residue. The precipitated solid was filtered, washed with n-hexane / ethyl acetate mixture (4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com