Clonidine hydrochloride sustained release micropill preparation
A technology of clonidine hydrochloride and sustained-release pellets, which can be used in cardiovascular system diseases, block delivery, drug combination, etc. The incidence of reaction, the effect of high and stable blood concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
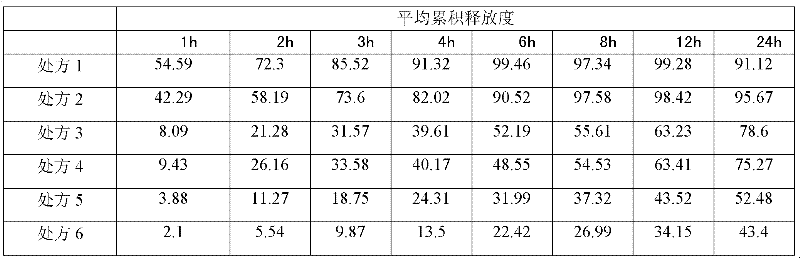
Examples
Embodiment 1
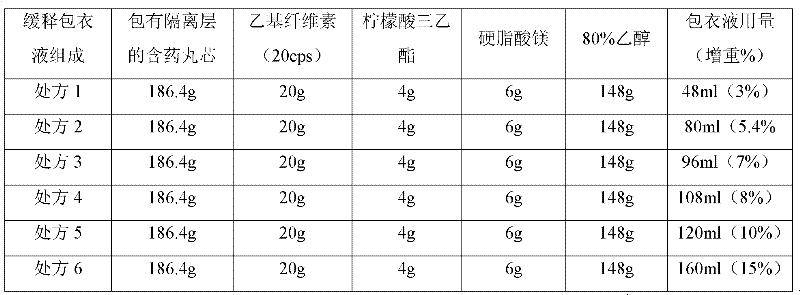
[0046] (1) Preparation of pill-containing core (A)
[0047] Clonidine hydrochloride
4.0g
Blank pellet core (sucrose type)
1600g
Opadry (CLEAN)
40.0g
purified water
760.0g
[0048] Preparation Process:
[0049] Weigh Opadry (CLEAN), add it to purified water and stir to dissolve, pass a 100-mesh sieve just before use, then add the prescription amount of clonidine hydrochloride raw material, stir and dissolve, to obtain a coating liquid. Weigh the blank pellets and add them to the fluidized bed coating machine (WBF-5G, Chongqing Yingge Granulation Coating Technology Co., Ltd.), and use the low spray coating system (Wurster system) for coating. The coating parameters of the equipment are :Inlet air volume 250m 3 / hr, inlet air temperature 50℃, atomization pressure 2kg / cm 2 , The flow rate of the coating liquid is 7rpm. After the coating liquid is sprayed, it is fluidized and dried for 10 minutes and then discharged to obtain a pill-containing core.
[0050] (2) Isol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap