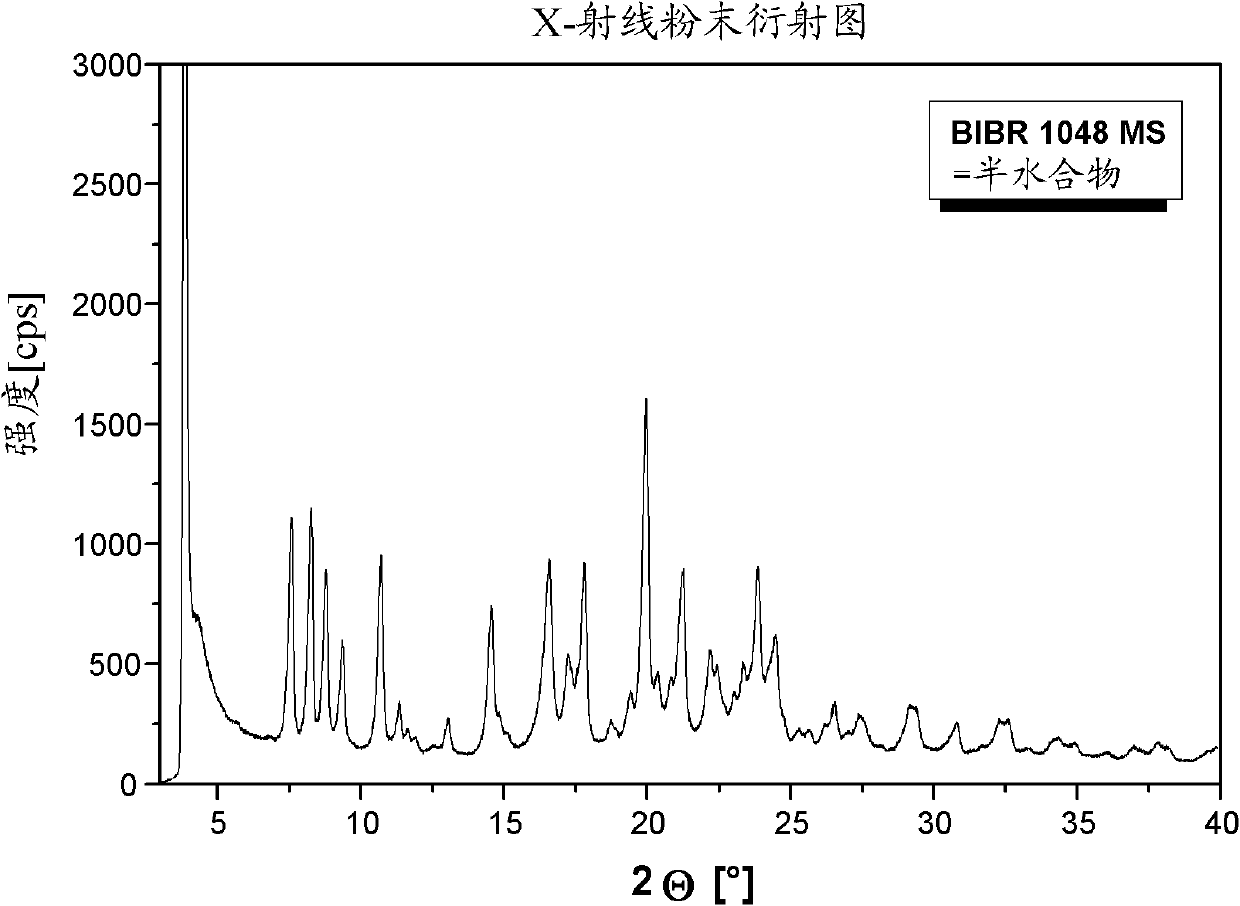
Benzimidazole-carbonyl-pyridine-amino-ehtyl-propionate hemihyrate and its use
A technology of benzimidazole and hemihydrate, which is applied in the field of benzimidazole carbonylpyridine allanine hemihydrate and its application field, and can solve the problems of harmful effects of drug reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
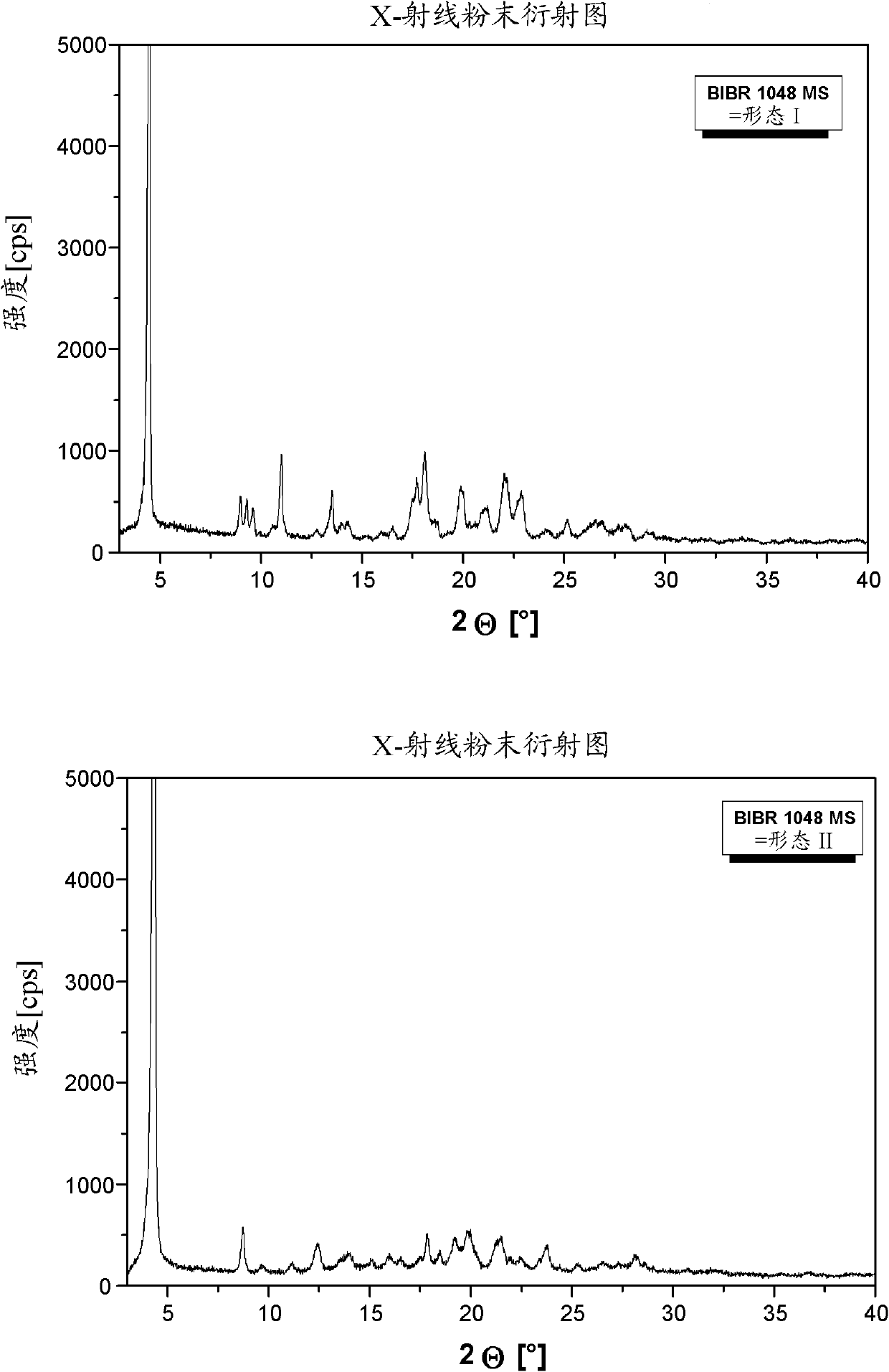
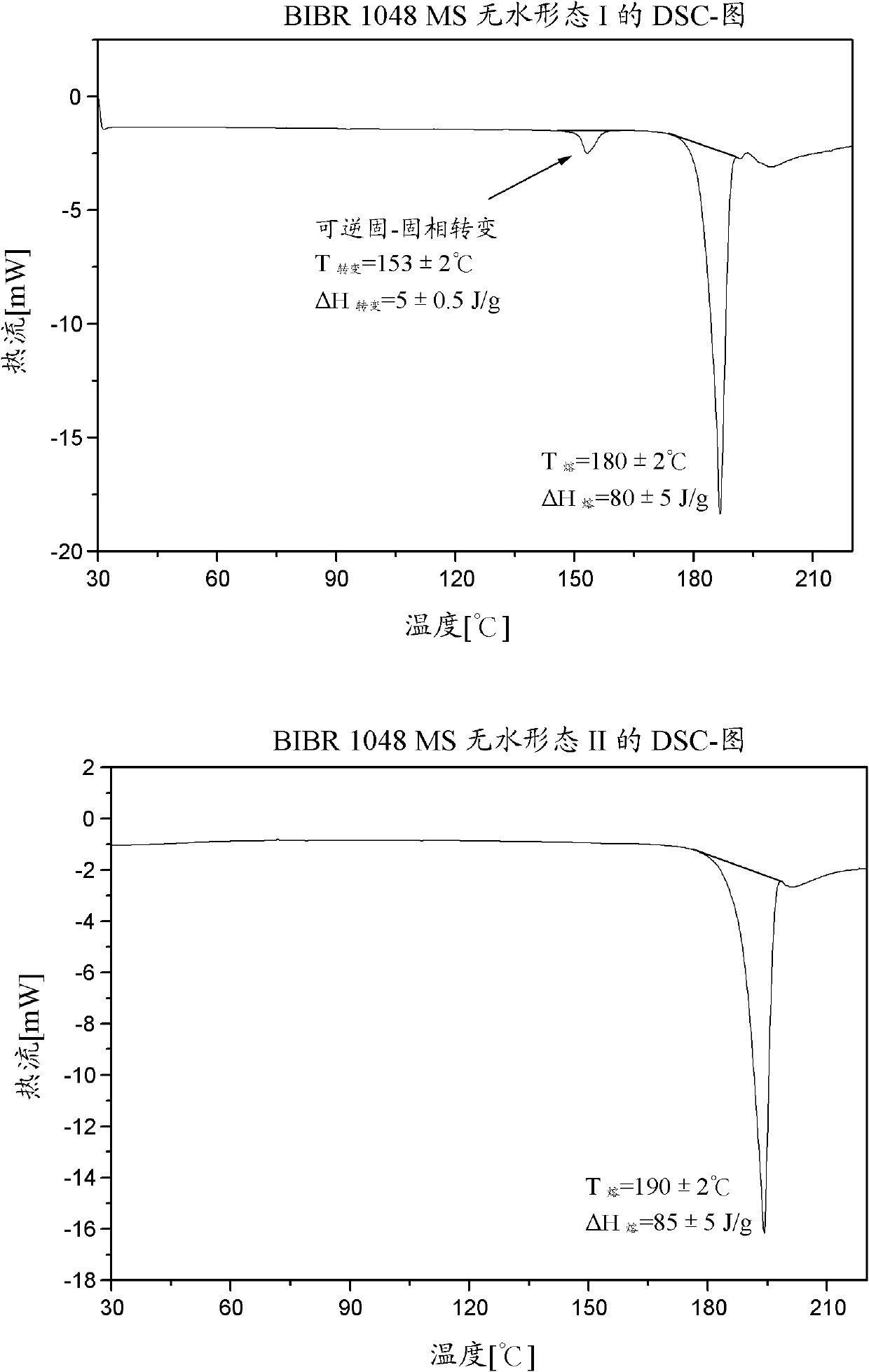
[0120] 3-[(2-{[4-(Hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1H-benzimidazole-5-carbonyl)-pyridine- 2-yl-amino]-propionic acid ethyl ester-mesylate salt form I (BIBR 1048 MS polymorph I)
[0121] 52.6 kg of 3-[(2-{[4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1H-benzimidazole-5-carbonyl) -Pyridin-2-yl-amino]-propionate ethyl ester base, which had preferably been previously purified by recrystallization from ethyl acetate, was placed in an inertized electric stirrer and 293 kg of acetone were subsequently added. The contents of the apparatus were heated to between 40 and 46°C with stirring. After a clear solution had formed, the contents of the device were filtered through a lens filter into a second electric stirring device and then cooled to between 30 and 36°C. 33 kg of acetone pre-cooled to between 0 and 5° C., 7.9 kg of 99.5% methanesulfonic acid and another 9 kg of acetone for rinsing were successively placed in the hanging...
Embodiment 2
[0124] BIBR 1048 MS polymorph II transformed from BIBR 1048 MS polymorph I
[0125] 4 grams of BIBR 1048 MS polymorph I and 35 milliliters of acetone were placed in a glass flask with a stirrer and reflux condenser. With stirring, the suspension was heated to between 45 and 50°C and kept at this temperature for 4 hours. It was then cooled to 15° C. and the crystals were filtered off with suction via a flat-bottomed (Büchner) funnel, washed with 20 ml of acetone and dried at 45° C. in vacuo.
[0126] Note: This synthesis can also be performed by seeding with BIBR 1048 MS polymorph II. If the conversion rate is slow, in addition to inoculating with BIBR 1048 MS polymorph II, it can be added by adding a small amount of BIBR1048 base (for example, on an industrial scale, about 50 grams of BIBR can be added to about 90 kg of BIBR 1048 MS polymorph I 1048 base) to accelerate it.
Embodiment 3
[0128] 3-[(2-{[4-(Hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1H-benzimidazole-5-carbonyl)-pyridine- 2-yl-amino]-propionic acid ethyl ester-mesylate salt form II (BIBR 1048 MS polymorph II)
[0129] 52.6 kg of 3-[(2-{[4-(hexyloxycarbonylamino-imino-methyl)-phenylamino]-methyl}-1-methyl-1H-benzimidazole-5-carbonyl) -Pyridin-2-yl-amino]-propionate ethyl ester base (preferably previously purified by recrystallization from ethyl acetate) was placed in an inertized electric stirrer and 293 kg of acetone were subsequently added. The contents of the apparatus were heated to between 40 and 46°C with stirring. After a clear solution had formed, the contents of the device were filtered through a lens filter into a second electric stirring device. 33 kg of acetone pre-cooled to between 0 and 5° C., 7.9 kg of 99.5% methanesulfonic acid and another 9 kg of acetone for rinsing were successively placed in the hanging container of the second device. At a temperature o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap