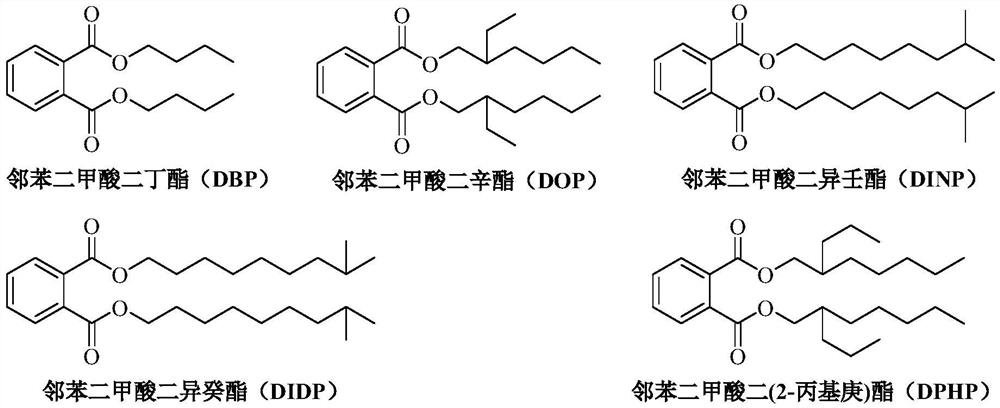
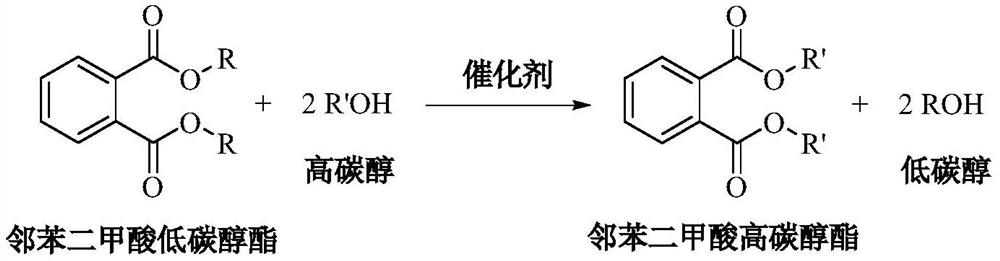
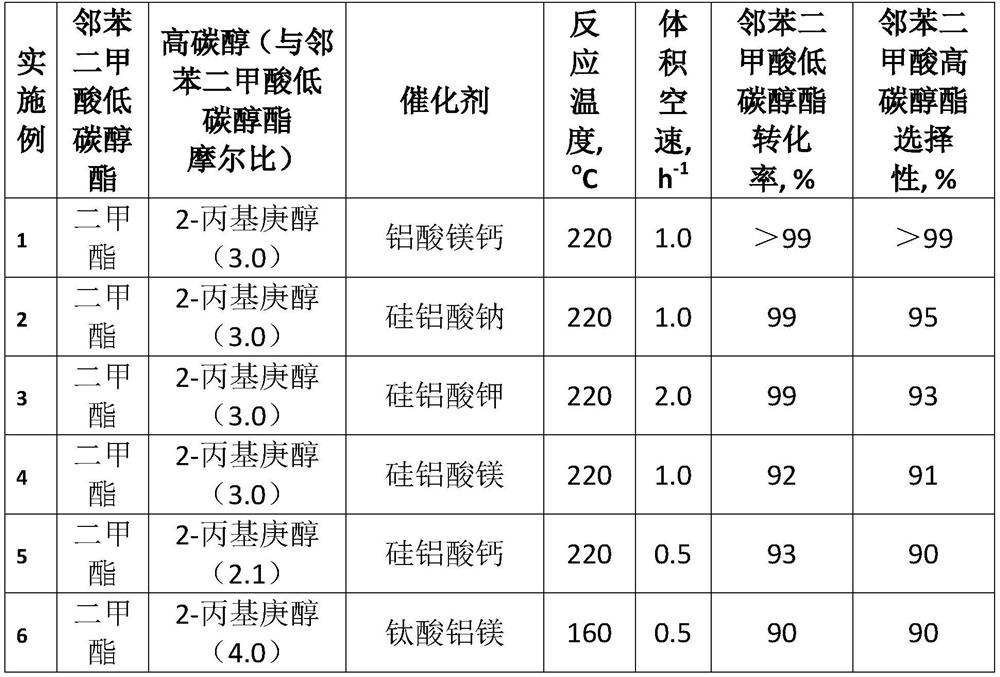
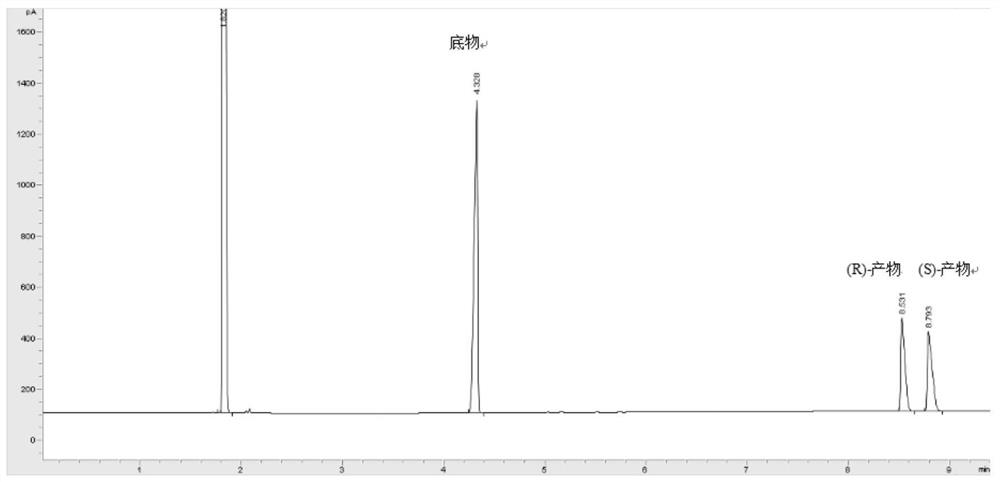
Patents
Literature
34 results about "Heptyl alcohol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Mixed alcohol fuels for internal combustion engines, furnaces, boilers, kilns and gasifiers
Mixed alcohol formulas can be used as a fuel additive in gasoline, diesel, jet fuel, aviation gasoline, heating oil, bunker oil, coal, petroleum coke or as a neat fuel in and of itself. The mixed alcohols formulations can contain C1-C5 alcohols, or in the alternative, C1-C8 alcohols or higher C1-C10 alcohols in order to boost energy content. The C1-C5 mixed alcohols contain more ethanol than methanol with declining amounts of propanol, butanol and pentanol. C1-C8 mixed alcohols contain the same, with declining amounts of hexanol, heptanol and octanol. C1-C10 mixed alcohols contain the same, with declining amounts of nananol and decanol. Synthetically produced mixed alcohol formulas feature higher octane and energy densities than either MTBE or fermented grain ethanol; more stable Reid Vapor Pressure blending characteristics; and increased soluablizing effects on condensate water. The primary benefits of mixed alcohols are increased combustion efficiencies, reduced emissions profiles and low production costs.
Owner:STANDARD ALCOHOL COMPANY OF AMERICA
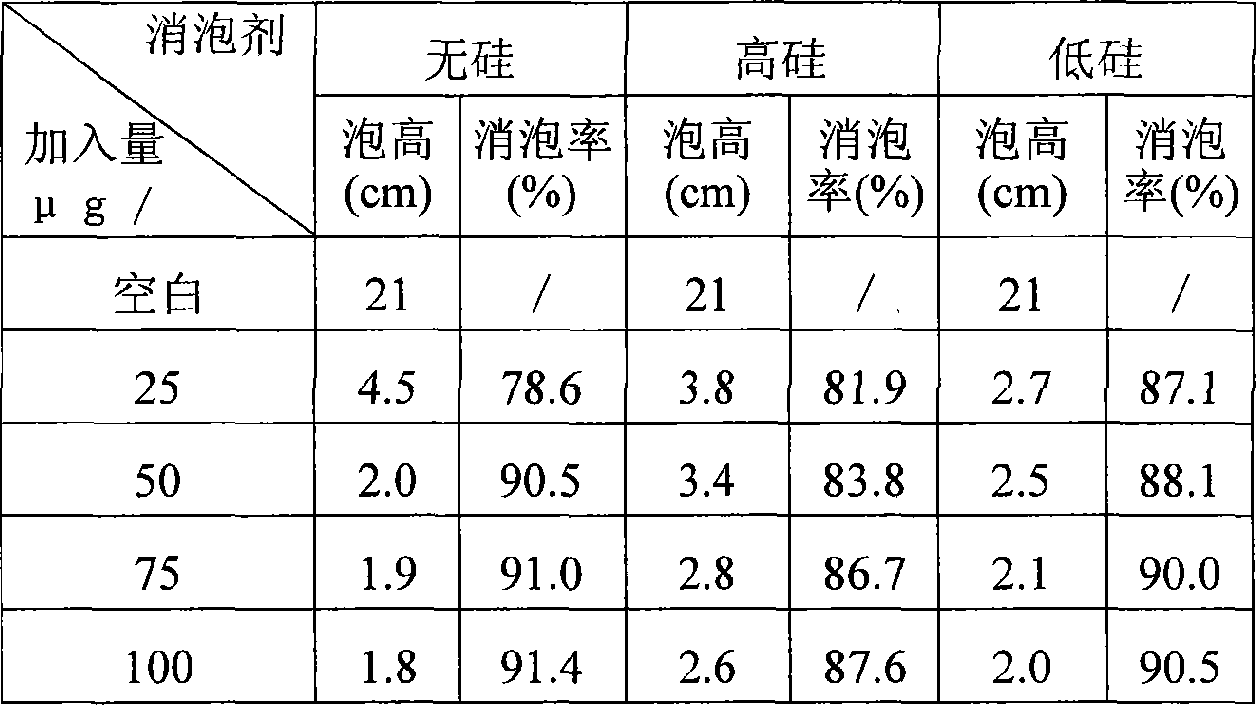
Additive for suppressing and delaying foam generation in coking reaction tower and its prepn process
InactiveCN101045880ASuppress and eliminate generationReduce entrainmentThermal non-catalytic crackingEpoxyHeptyl alcohol
The present invention is additive for suppressing and delaying foam generation in coking reaction tower and its preparation process, and relates to oil refining additive technology. The additive is prepared through compounding high molecular weight block polyether 30-50 wt%, fatty alcohol 20-40 wt% and solvent 20-40 wt%, where, the high molecular weight block polyether is block copolymer of ethylene glycol, epoxy ethane and epoxy propane of molecular weight 10,000-20,000, and the fatty alcohol is isocotanol or 3-enanthol, and the solvent is water solution of alcohol. The preparation process includes the first feeding solvent into reaction kettle and heating slowly to 40-60 deg.c, adding block polyether and fatty alcohol in the ratio via stirring to react for 1-2 hr, cooling and filtering off solid impurity to obtain the product. The additive can suppress and delay foam generation in coking reaction tower effectively.
Owner:SHENYANG POLYTECHNIC UNIV
Mixed alcohol fuels for internal combustion engines, furnaces, boilers, kilns and gasifiers
Mixed alcohol formulas can be used as a fuel additive in gasoline, diesel, jet fuel, aviation gasoline, heating oil, bunker oil, coal, petroleum coke or as a neat fuel in and of itself. The mixed alcohols formulations can contain C1-C5 alcohols, or in the alternative, C1-C8 alcohols or higher C1-C10 alcohols in order to boost energy content. The C1-C5 mixed alcohols contain more ethanol than methanol with declining amounts of propanol, butanol and pentanol. C1-C8 mixed alcohols contain the same, with declining amounts of hexanol, heptanol and octanol. C1-C10 mixed alcohols contain the same, with declining amounts of nananol and decanol. Synthetically produced mixed alcohol formulas feature higher octane and energy densities than either MTBE or fermented grain ethanol; more stable Reid Vapor Pressure blending characteristics; and increased soluablizing effects on condensate water. The primary benefits of mixed alcohols are increased combustion efficiencies, reduced emissions profiles and low production costs.
Owner:STANDARD ALCOHOL COMPANY OF AMERICA
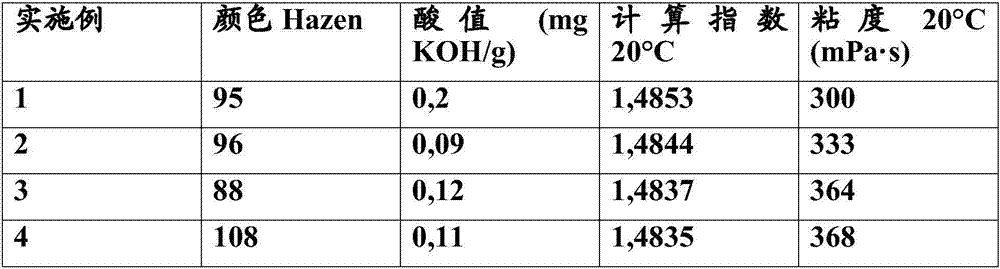
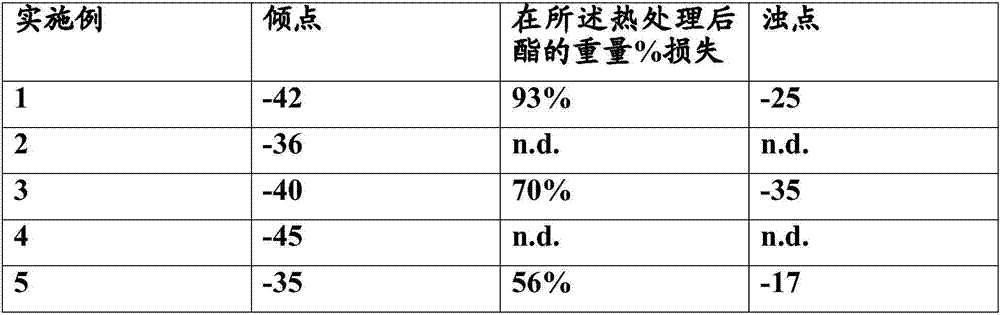
Ester plasticizers based on ethylhexanol and propylheptanol
The present invention relates to an ester obtainable by reacting (a) an alcohol mixture comprising 20-50 wt% ethylhexanol, 40-64 wt% propylheptanol and 0-20 wt% of other alcohols with (b) a polycarboxylic acid. Further aspects of the invention relate to a thermoplastic polymer comprising the ester according to the invention and uses of said ester.
Owner:EMERY OLEOCHEMICALS GMBH
Methanol diesel oil cleaning grade-raising emulsifier for vehicle
the invention discloses a cleaned standard-increasing emulsifier of vehicle carbinol gasoline, which is characterized by the following: loading positive octyl alcohol, positive heptyl alcohol, alcohol isopropylicum, positive butyl alcohol, positive decatyl alcohol, hexadecyl alcohol and spans 80 in the autoclave according certain weight percentage; stirring evenly under the condition of normal temperature without any fire.
Owner:段家祥
Water-soluble high-temperature organic acid inhibitor and preparing method and using method thereof
The present invention relates to a high-temperature organic acid corrosion inhibitor for inhibiting refinery vacuum tower high-temperature naphthenic acid corrosion environment with 200-400deg.C. Its composition includes (by wt%) 10-60% of corrosion inhibitor intermediate obtained by using boric acid and organic amine according to the mole ratio of 1:0.5-3 through a certain reaction process, 20-80% of monohydric alcohol or dihydric alcohol solvent formed from one kind and two kinds of methyl alcohol, ethyl alcohol, n-propyl alcohol, isopropyl alcohol, butyl alcohol, isobutyl alcohol, 1,2-propylene glycol, n-amyl alcohol, n-heptyl alcohol and n-caprylic alcohol or combination of more than two kinds of above-mentioned components and 0.1-20% of corrosion inhibition film-forming component formed from one kind or any two kinds of sulfourea, benzyl chloride, alkylene sulfide, propiolic alcohol, trisodium phosphate, ammonium phosphosulfate, diammonium phosphate, triammonium phosphate and benzotriazole or combination of more than two kind of above-mentioned all the components.
Owner:PETROCHINA CO LTD
Composition for odour improvement
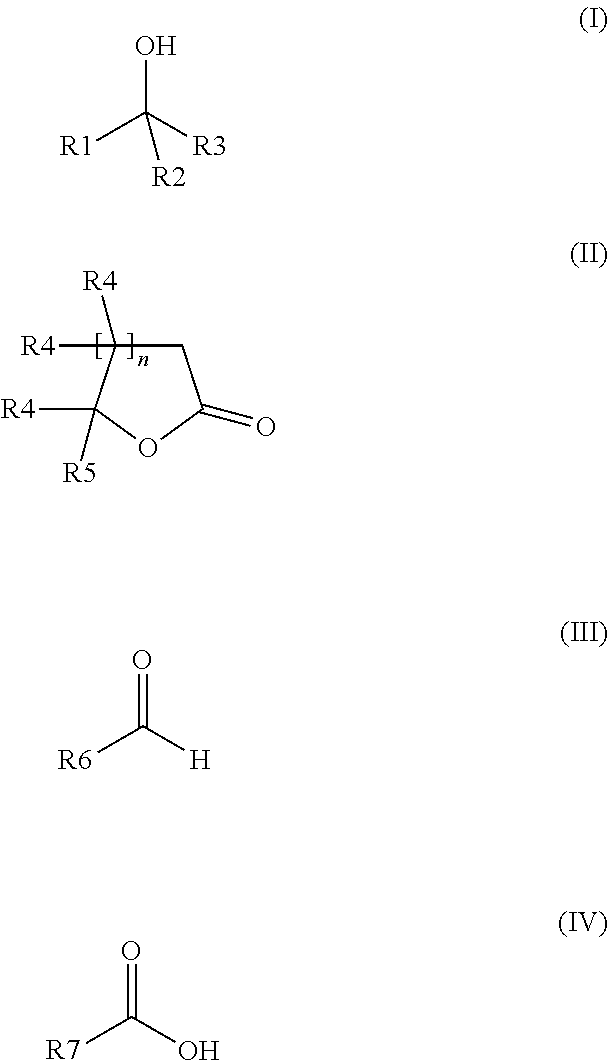
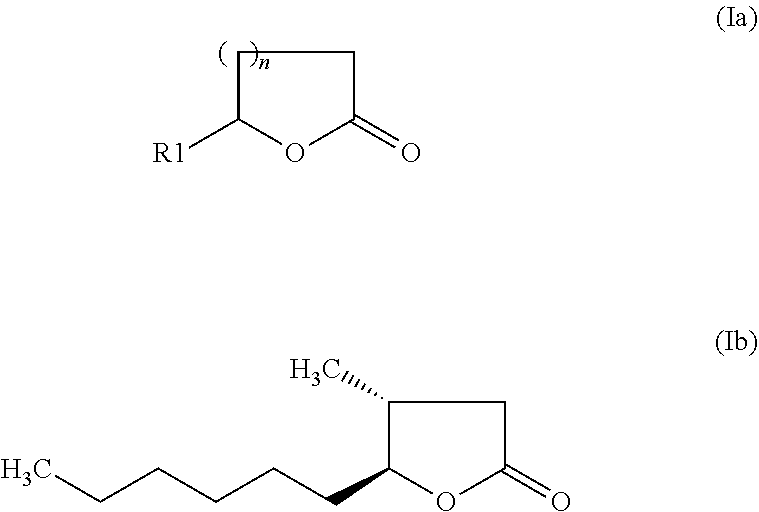
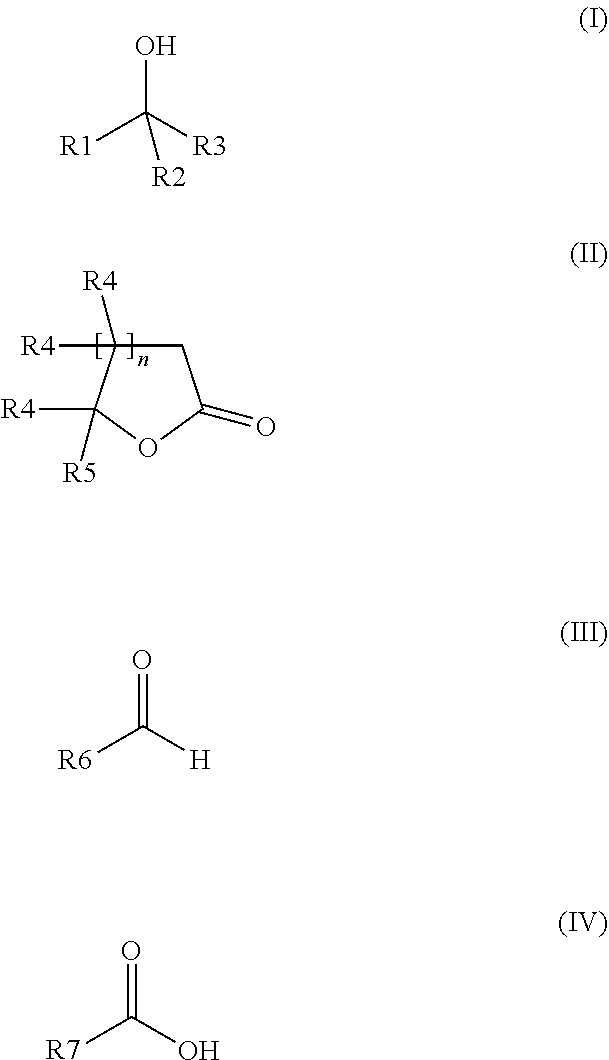
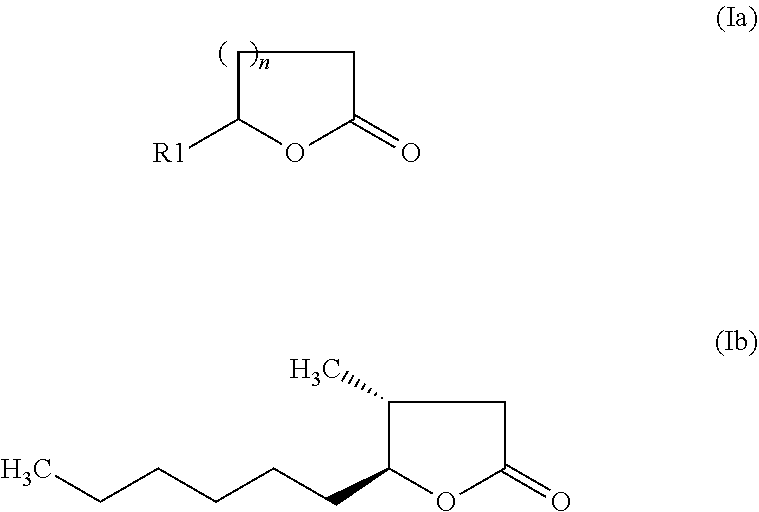
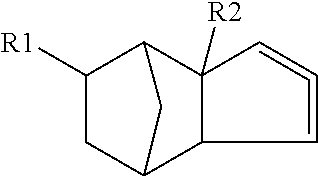
The invention relates to a preparation containing: (i) a composition containing (a) one, two or a plurality of compounds selected from the group consisting of (a1) alcohol monoterpenes of formula (I) in which R1 is H or methyl, R2 is H or C2-alkenyl, and R3 is a linear or branched, saturated or unsaturated hydrocarbon radical with 4 to 10 carbon atoms, and the enantiomers, diastereomers, racemates, solvates and physiologically compatible salts thereof, and / or (a2) bicyclic epoxy-monoterpenes, (b) at least two lactones of formula (II) in which R4 is H or methyl, R5 is a linear or branched, saturated or unsaturated hydrocarbon radical with 2 to 10 hydrocarbon atoms and n is the number 1 or 2, and the enantiomers, diastereomers and racemates thereof, (c) one, two or a plurality of solvents selected from the group consisting of ethanol, water, dipropylene glycol (DPG), diethyl phtalate (DEP), propylene glycol (PG), isopropyl myristate (IPM), isopropyl palmitate (IPP), triethyl citrate (TEC), triacetin (TRI), 1,2-Propanediol, 1,3-Propanediol, Propanethiol, Pentanediol, Hexanediol, Octanediol, Decanediol (SymClariol®), Dodecanol, 4-hydroxy-acetophenone (SymSave® H), glycerine, butylene glycol, pentylene glycol, hexylene glycol, decylene glycol, propylene carbonate, butylene carbonate, glycerine carbonate, 2-5 benzyl heptanol, lauryl alcohol, trimethyl-hydroxypentyl-isobutyrate, glyceryl-caprylate, ethylhexyl glycerine, benzyl benzoate (BB), and optionally (d) other flavouring agents or aromatic substances selected from the group consisting of 3-phenylbutanal (Trifernal), acetyl methyl carbinol, anethole, anisyl acetate, dihydroeugenol, linalyl formate, 2-methyldecanal, 2-benzyl-2-methylbut-3-ene nitrile (Ci-trowanil® B), 3-hexenyl acetate, styrallyl acetate, belanis, citronellal, cinnamyl acetate, rhubafuran, beta-ions, anther, prenyl acetate, 2-phenyl propanal, 4-(4-hydroxyphenyl)butan-2-one (Frambinon®), ethyl phenoxyacetate, isoralderine, gamma-terpinene, limonene, neocyclocitral, methyl lavender ketone, styrallyl propionate, phenyl ethyl propionate, limonenal, 4-isopentylcyclohexanol (Symrose®), 4-methyl-2-phenyl-3,6-dihydro-2H-pyran / 4-methylene-2-phenyl-tetrahydropyrane (Rosyrane super), hydrocitronitril, phenoxanol, isoamyl phenylacetate, damascone, silvial, nectaryl, ambroxide, acetyl pyrazine, trimethyl pyrazine, isoamyl acetate, para-cresyl methyl ether, filbertone, cyclohexyl acetate, heliotropin, acetophenone, anisaldehyde, para-methyl acetophenone, veratraldehyde, methyl anisate and vertoprenal; (ii) aldehydes of formula (III) in which R6 is a saturated or non-saturated, linear hydrocarbon radical; and / or (iii) free fatty acids of formula (IV), in which R7 is a linear or branched, saturated hydrocarbon radical.
Owner:SYMRISE GMBH & CO KG
Methanol gasoline cleaning grade-raising emulsifier for vehicle
the invention discloses a cleaned standard-increasing emulsifier of vehicle carbinol gasoline, which is characterized by the following: loading positive octyl alcohol, positive heptyl alcohol, alcohol isopropylicum, positive butyl alcohol, positive decatyl alcohol, hexadecyl alcohol and spans 80 in the autoclave according certain weight percentage; stirring evenly under the condition of normal temperature without any fire.
Owner:烟台远弘能源科技有限公司
Black soybean milk flavor and preparation method and application thereof
The invention discloses a black soybean milk flavor and a preparation method and an application thereof. The flavor comprises isovaleric aldehyde, methyl alcohol, isopentyl alcohol, hexanal, 2-methylpyrazine, furfural, trans-2-hexenal, furfuryl alcohol, hexyl alcohol, 2-heptanone, heptanal, 2-acetylfuran, 2, 5-methylpyrazine, 2, 3-dimethyl pyrazine, trans-2-heptenal, 5-methyl-furfural, benzaldehyde, heptanol, 1-octen-3-alcohol, 2-pentyl-furan, caprylaldehyde, 2-acetyl thiazole, trans-2-octenyl aldehyde, octanol, nonanal, capraldehyde, trans,trans-2,4-nonadienal, trans-2-decenal, 2-undecanone,trans,trans-2,4-heptadienal, trans-2-undecenal and propylene glycol. The method comprises the following steps: evenly mixing the above constituents, stewing and curing for 15 days, subpackaging to obtain the black soybean milk flavor. The flavor has convenient raw materials sources, mild fragrance and high naturalness, and is applied to preparing the beans products, is especially used for preparing black soybean milk beverage, not only can enhance mouthfeel, but also can better cover soil mildewed flavor brought by beans in basic materials.
Owner:广州市凯虹香精香料有限公司
Vehicle methanol gasoline cosolvent
ActiveCN103695052AImprove stabilityStrong solubility aidLiquid carbonaceous fuelsDiacetone alcoholHeptyl alcohol
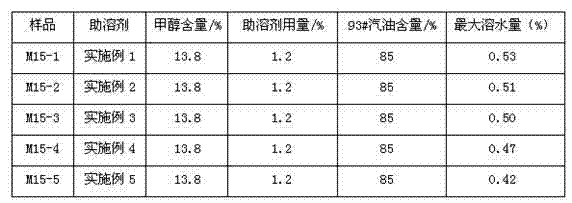
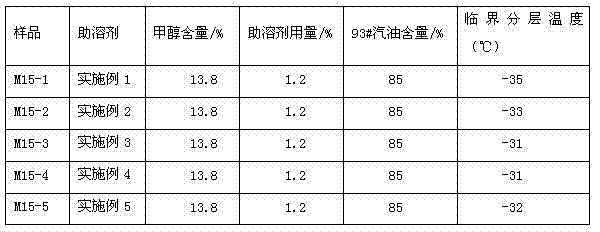
The invention belongs to the technical field of vehicle fuel oil additives, and relates to efficient vehicle methanol gasoline cosolvent which is a mixture comprising the following seven different alcohols: 3-20 parts of ethylene glycol monobutyl ether oleate, 2-5 parts of diacetone alcohol, 4-10 parts of glycol monoethyl ether, 10-80 parts of butanol, 5-50 parts of pentanol, 5-20 parts of hexanol, 0.5-10 parts of heptanol and 0.5-10 parts of octanol. According to the invention, the cosolvent has high hydrotropy; prepared methanol gasoline can be stored for long time under a low-temperature condition of -30 DEG C, and the water resistance capability is up to 0.5%; and the efficient cosolvent is free of metal and other elements, is reasonable in element variety and has no adverse influence on an engine, and the combustion performance of the cosolvent is approximate to that of gasoline. The efficient vehicle methanol gasoline cosolvent is a brand new technology and product in the field of methanol gasoline, and is of far reaching importance in improving the quality of methanol gasoline and standardizing industrial additive technologies.
Owner:YANKUANG GRP CO LTD +1
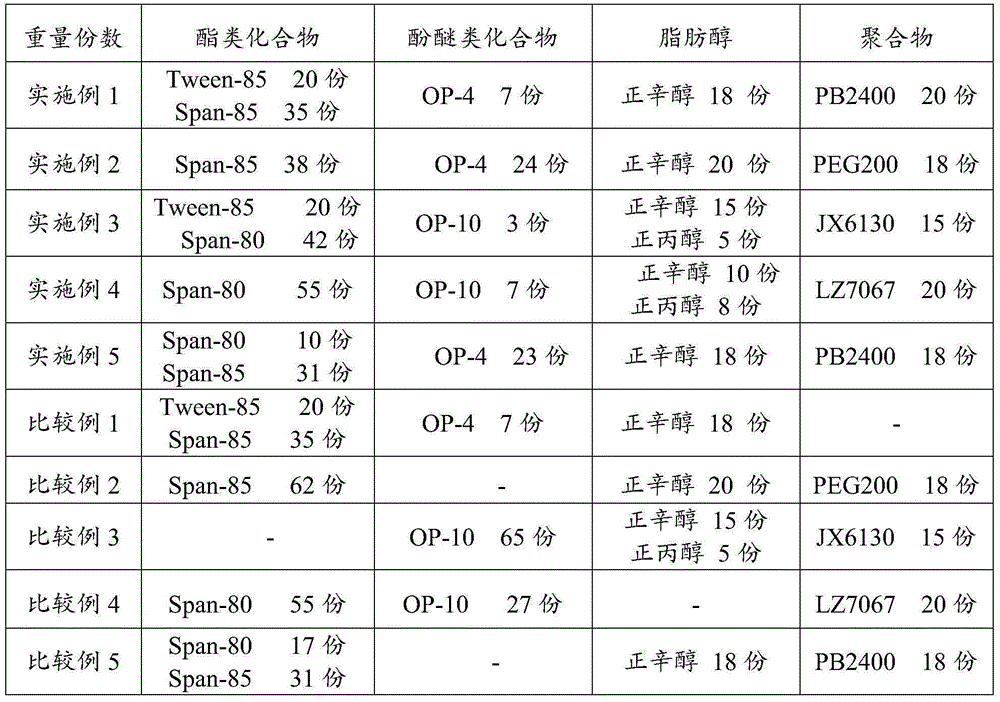
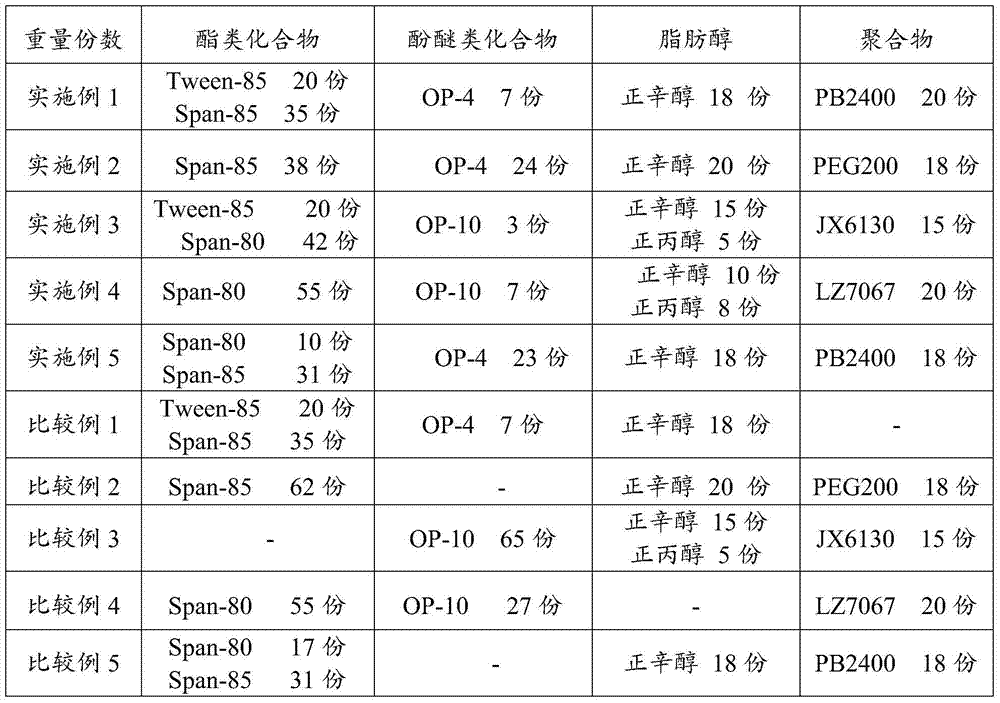
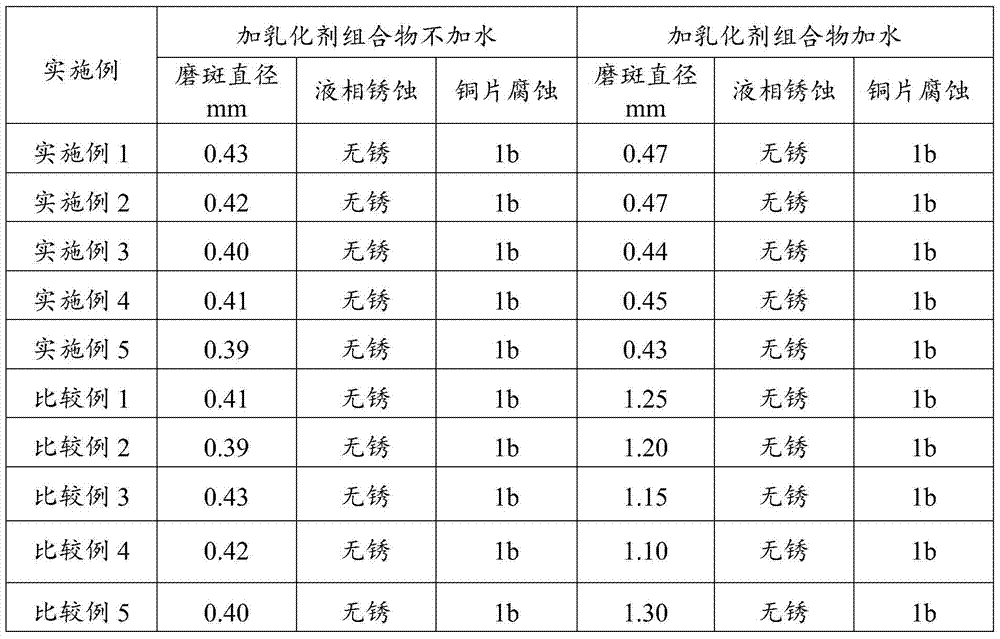
Marine oil emulsifier composition and application thereof
InactiveCN105238460AGood emulsifying effectAffect extreme pressure and wear resistanceLiquid carbonaceous fuelsN-Propyl alcoholPolyethylene glycol
The invention relates to a marine oil emulsifier composition and application thereof which mainly solve the problem that in the prior art, system oil for a screw shaft cannot form stable emulsifying oil when the system oil encounters water. The marine oil emulsifier composition is prepared from, by weight, 0.1-80 parts of ester compounds, 0.1-50 parts of phenolic ether compounds, 0.1-50 parts of fatty alcohol and 0.1-50 parts of polymers; at least one of sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester and polyethylene glycol fatty acid ester is selected as the ester compounds, at least one of a TX series and an OP series is selected as the phenolic ether compounds, at least one of methyl alcohol, ethyl alcohol, n-propyl alcohol, n-butyl alcohol, n-amyl alcohol, n-hexyl alcohol, n-heptanol, n-caprylic alcohol and isooctyl alcohol is selected as the fatty alcohol, and at least one of polyethylene glycol, polyisobutene and ethylene-propylene copolymers is selected as the polymers. According to the marine oil emulsifier composition and the application thereof, the problem is better solved, and the marine oil emulsifier composition can be applied to industrial production.
Owner:CHINA PETROLEUM & CHEM CORP +1
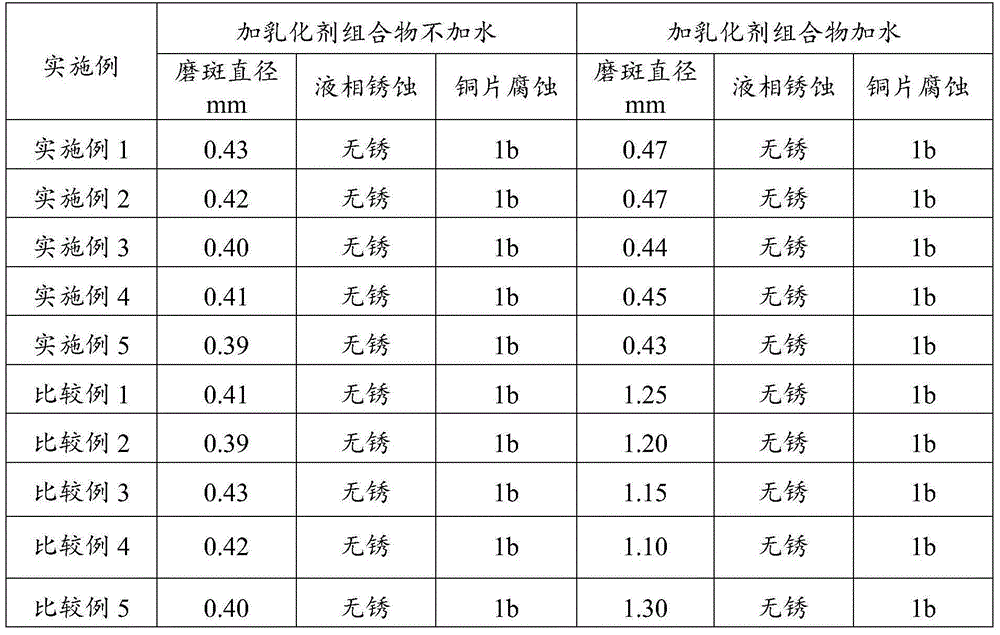
Bunker oil emulsifier composition and applications thereof
ActiveCN104263464AGood emulsifying effectAffect extreme pressure and wear resistanceAdditivesN-HeptanolPolyethylene glycol
The invention relates to a bunker oil emulsifier composition and applications thereof, which are mainly designed for solving the problem that in the prior art, system oil applied to screw shafts, after meeting water, can not form stable emulsified oil. The bunker oil emulsifier composition disclosed by the invention comprises the following components in parts by weight: a) 0.1-80 parts of an ester compound, b) 0.1-50 parts of a phenolic ether compound; c) 0.1-50 parts of fatty alcohol, and d) 0.1-50 parts of a polymer; and a technical scheme that the ester compound is selected from at least one of sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester or poly-ethylene glycol fatty acid ester, the phenolic ether compound is selected from at least one of TX series or OP series, the fatty alcohol is selected from at least one of methanol, ethanol, n-propanol, n-butanol, n-amyl alcohol, n-hexanol, n-heptanol, n-octanol or 2-ethylhexanol, and the polymer is selected from at least one of polyethylene glycol, polyisobutylene or ethylene-propylene copolymer solves the problem well. The bunker oil emulsifier composition can be applied to the industrial production of bunker oil emulsifier compositions.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthetic process for DOTP-1
InactiveCN103664621AShort reaction timeLow reaction temperatureOrganic compound preparationCarboxylic acid esters preparationHeptyl alcoholReaction temperature
The invention discloses a synthetic process for DOTP-1. The synthetic process comprises the following steps: (1) taking raw materials at the molar ratio of terephthalic acid: heptyl alcohol=1:(2.7-2.9), and taking 3.3% of the total mass of phthalic acid and heptyl alcohol of a titanium(IV) isopropoxide catalyst; (2) putting all terephthalic acid and one third of the heptyl alcohol into a reaction kettle, heating up to 150-180 DEG C, adding 50% of the total quantity of the catalyst, and adding the catalyst within 4-6 minutes; (3) heating a reaction liquid I obtained in step (2) up to 190-210 DEG C, adding one third of the heptyl alcohol, and adding 25% of the catalyst after 5-10 minutes; (4) conducting heat preservation on a reaction liquid II obtained in step (3), adding surplus heptyl alcohol and catalyst into the reaction liquid II for reacting to obtain a crude product; (5) conducting water washing, dealcoholization, decoloring and filtering on the crude product to obtain a finished product. According to the synthetic process, the reaction time is greatly shortened and the reaction temperature is greatly lowered, so that the product quality is guaranteed and the product grade and yield are also improved, and the energy consumption and manufacturing cost are lowered.
Owner:ZHEJIANG HAILIYE TECH
Prepn of fatty alcohol acetate
InactiveCN1436765AImprove efficiencySolve boiling pointPreparation by ester-hydroxy reactionHeptyl alcoholReaction temperature
The present invention is the preparation process of fatty alcohol acetate with methyl acetate and fatty alcohol as material. In the presence of catalyst, methyl acetate and fatty alcohol are made to produce ester exchange reaction at the reaction temperature of 70-180 deg.c and the reaction pressure of 0.15-2.0 MPa for 2-6 hr before the reaction material is rectified and separated to obtain product fatty alcohol acetate. The fatty alcohol may one of ethanol, propyl alcohol, isopropyl alcohol, butyl alcohol, isobutyl alcohol, amyl alcohol, isoamyl alcohol, hexyl alcohol, isohexyl alcohol, heptyl alcohol, isoheptyl alcohol, capryl alcohol and isocapryl alcohol. The present invention solves the technological problem that methyl acetate has boiling point lower than that of other matter in the ester exchange system and thus can not produce ester exchange reaction with other fatty alcohol.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Methanol gasoline cleaning grade-raising emulsifier for vehicle
Owner:烟台远弘能源科技有限公司
Method for determining floral components in mainstream smoke of cigarettes
The invention belongs to the technical field of smoke component detection, and particularly relates to a method for determining floral components in mainstream smoke of cigarettes. The method comprises the following steps: extracting collected cigarette mainstream smoke particulate matters by using acetone to obtain an extracting solution; analyzing the extracting solution by adopting gas chromatography-mass spectrometry, and detecting the content of the floral components by adopting an internal standard method, wherein an internal standard substance used in the internal standard method is storax propionate, and the floral component is composed of heptanol, sec-octyl alcohol, p-toluenemethyl ether, 2-ethylhexanol, ocimene, phenylacetaldehyde and other components with floral fragrance. Themethod disclosed by the invention can be used for simultaneously detecting the contents of the 45 floral components in the cigarette smoke, and has the advantages of rapid detection, high sensitivity,good selectivity and high accuracy.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Stable fuel additive
InactiveCN104449911AChemically stableIncrease profitLiquid carbonaceous fuelsHeptyl alcoholN-Propyl alcohol
The invention relates to a stable fuel additive, which is prepared from the following raw materials in parts by weight: 6-11 parts of ethylene glycol methyl ether, 6-8 parts of isobutyl alcohol, 7-10 parts of a nanoparticle magnetic solution, 3-9 parts of sorbitan monooleate, 4-7 parts of normal propyl alcohol, 6-9 parts of dibutyl hydroxytoluene, 3-8 parts of dicyclohexylamine nitrite, 5-7 parts of n-heptanol, 2-6 parts of n-butyl alcohol, 2-5 parts of OP-15, 1-5 parts of a dispersing agent, 3-5 parts of methylsilicone oil and 3-7 parts of linoleic acid. The stable fuel additive provided by the invention has the beneficial effects of being stable in chemical property and not easy to go bad; meanwhile, the use ratio of the fuel is improved; the resource waste is reduced; the stable fuel additive has an excellent economic value.
Owner:QINGDAO KANGTAIXIN ENVIRONMENTAL PROTECTION TECH
Methanol diesel oil cleaning grade-raising emulsifier for vehicle
InactiveCN100392049CIncrease motivationEasy to drainLiquid carbonaceous fuelsHeptyl alcoholAutomotive engine
the invention discloses a cleaned standard-increasing emulsifier of vehicle carbinol gasoline, which is characterized by the following: loading positive octyl alcohol, positive heptyl alcohol, alcohol isopropylicum, positive butyl alcohol, positive decatyl alcohol, hexadecyl alcohol and spans 80 in the autoclave according certain weight percentage; stirring evenly under the condition of normal temperature without any fire.
Owner:段家祥
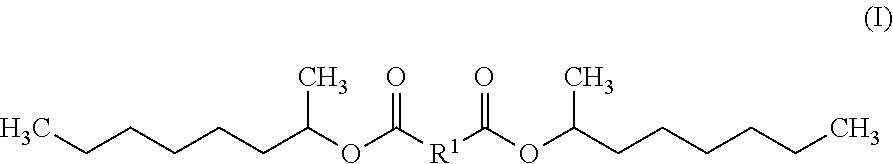
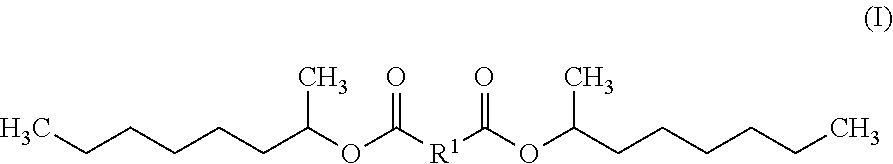
Diesters for Personal Care Applications Derived from 1-Methylheptyl Alcohol
ActiveUS20170291868A1Altering tactile impressionAltering skinfeelCosmetic preparationsMake-upPersonal careAlcohol
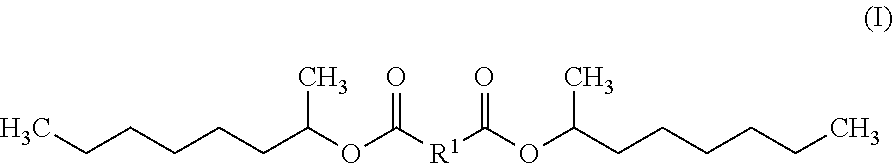
The invention includes a diester exhibiting improved hydrolytic stability that is an esterification product of a 1-methylheptyl alcohol and a dicarboxylic acid. In some embodiments, the diester is bis(1-methyl heptyl) butanedioate, bis(1-methyl heptyl) nonanedioate and / or bis(1-methyl heptyl) decanedioate. The diester may be natural and / or not sourced from palm. Also contemplated within the scope of the invention are diesters exhibiting improved hydrolytic stability represented by Formula (I):wherein R1 is chosen from a linear alkyl group containing four to ten carbon atoms. Also included are personal care compositions comprising any of these diesters and methods of preparing a personal care composition using the inventive diesters, and / or methods of altering the tactile impression and / or skinfeel provided to a user by a personal care composition by combining any of the diesters of the invention and at least one personal care component to form a personal care composition; and topically applying the personal care composition to the hair, skin, and / or nails of a user.
Owner:INOLEX INVESTMENT CORP
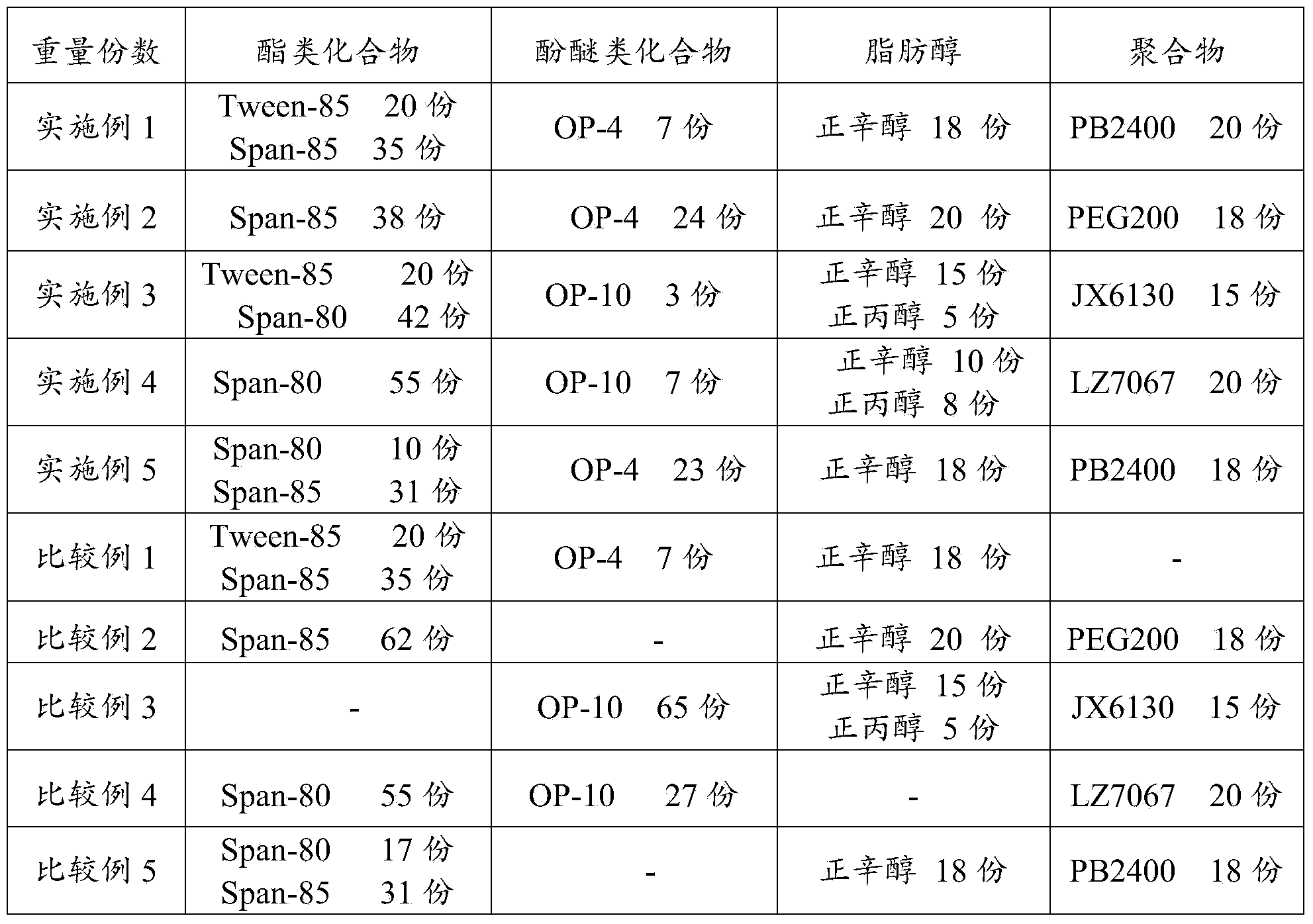
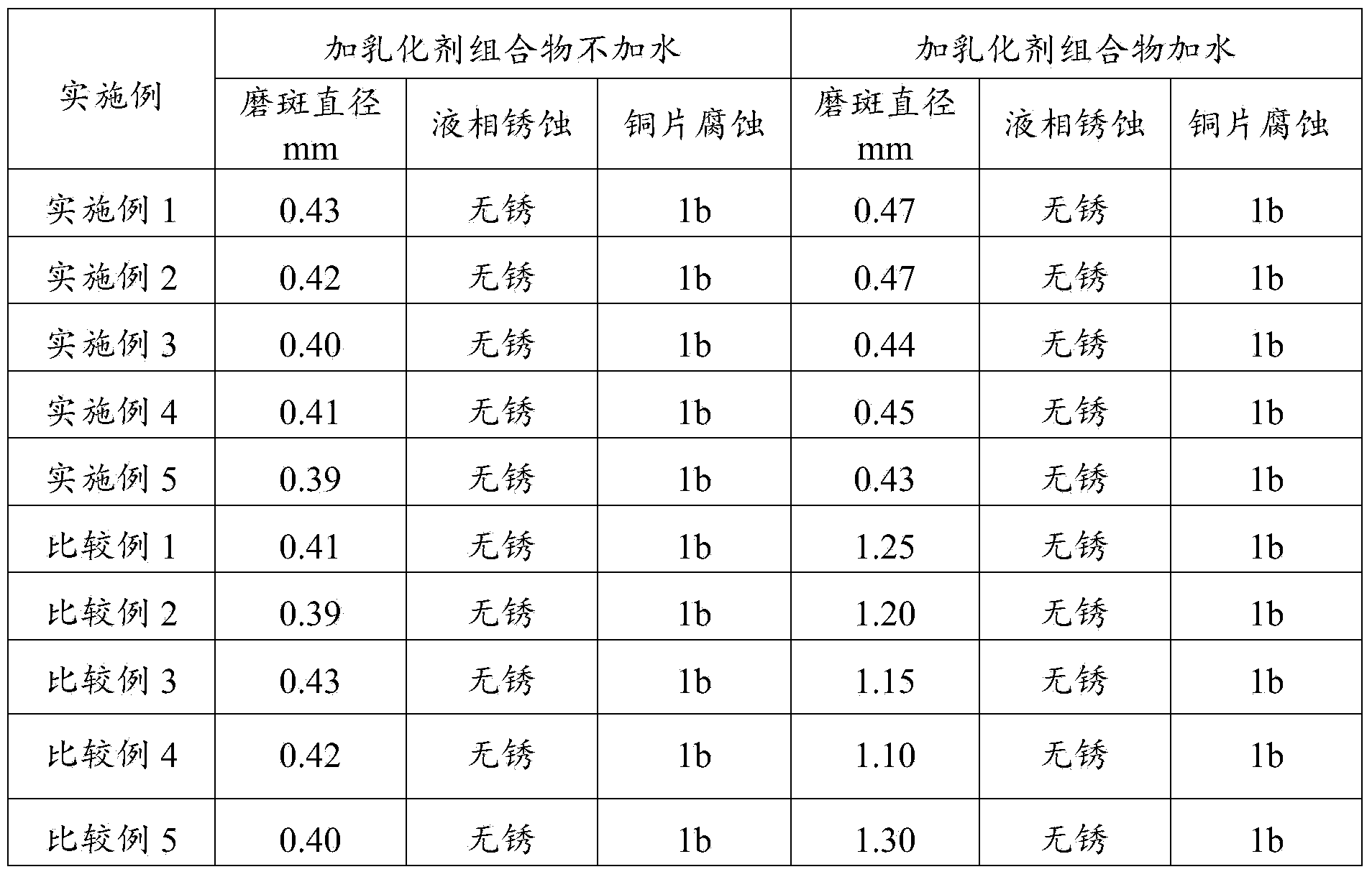
Bunker oil emulsifier composition and use thereof
ActiveCN104263464BGood emulsifying effectGood extreme pressure and anti-wear propertiesAdditivesHeptyl alcoholPolymer science
The invention relates to a marine oil emulsifier composition and its application, which mainly solves the problem in the prior art that the system oil used in the stern shaft cannot form stable emulsified oil when it encounters water. The present invention includes the following components in parts by weight: a) 0.1-80 parts of ester compounds; b) 0.1-50 parts of phenol ether compounds; c) 0.1-50 parts of fatty alcohols; d) 0.1-50 parts of polymer The ester compound is selected from at least one of sorbitan fatty acid ester, polyoxyethylene sorbitan fatty acid ester or polyethylene glycol fatty acid ester; the phenolic ether compound is selected from TX At least one of series or OP series; The fatty alcohol is selected from at least one of methanol, ethanol, n-propanol, n-butanol, n-pentanol, n-hexanol, n-heptanol, n-octanol or isooctyl alcohol The technical scheme that said polymer is selected from at least one of polyethylene glycol, polyisobutylene or ethylene-propylene copolymer solves this problem well, and can be used in the industrial production of the bunker oil emulsifier composition.
Owner:CHINA PETROLEUM & CHEM CORP +1
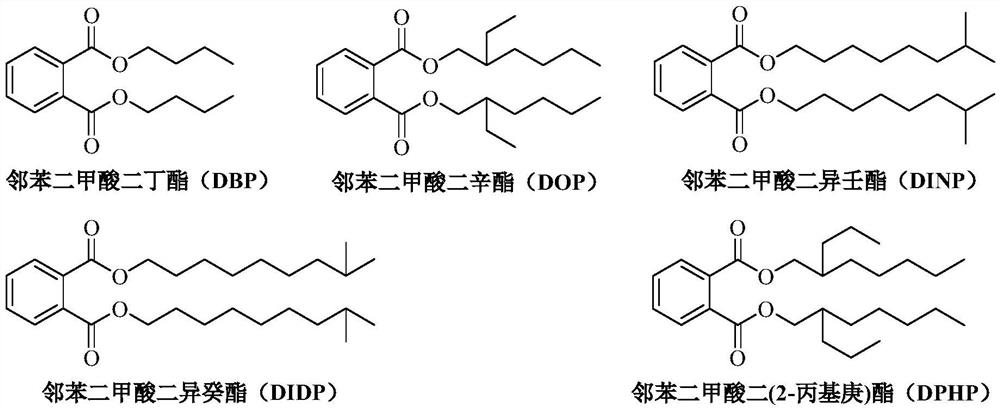
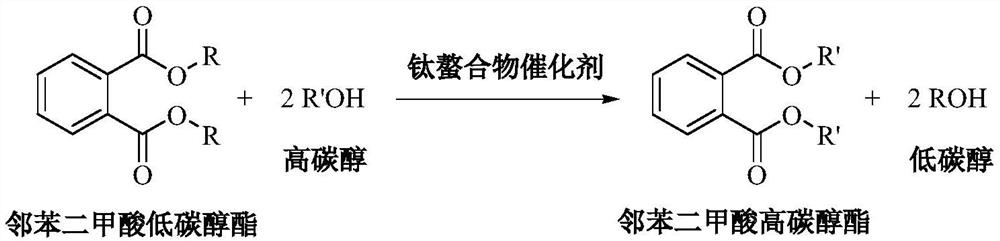
A kind of method for transesterification continuous reaction to prepare higher carbon alcohol phthalate
ActiveCN108976117BGood storage and transportation stabilityReduce consumptionPreparation by ester-hydroxy reactionHeterogenous catalyst chemical elementsHeptyl alcoholPtru catalyst
The invention relates to a method for preparing higher alcohol phthalate through continuous transesterification reaction. In a fixed-bed reactor, dimethyl phthalate, diethyl phthalate, diphthalate Low-carbon alcohols such as butyl phthalate and high-carbon alcohols such as isononanol, isodecyl alcohol, and 2-propylheptanol are used as raw materials, and magnesium calcium aluminate, sodium aluminosilicate, potassium aluminosilicate, silicon One or more of magnesium aluminate, calcium aluminosilicate, aluminum magnesium titanate, magnesium calcium titanate, and aluminum magnesium zirconate are catalysts, and the molar ratio of higher alcohol to lower alcohol phthalate is 2.1-4.0 , reaction temperature 160~220℃, volume space velocity 0.5~2h ‑1 , The conversion rate of low-carbon alcohol phthalate and the selectivity of high-carbon alcohol phthalate are above 99%. The invention has the following advantages: good stability of raw material storage and transportation, convenient feeding; less side reactions, low raw material consumption; no corrosion of the catalyst and continuous use; clean and energy-saving process, no waste water generation and discharge; reaction in a fixed bed reactor Continuous, high efficiency.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Prepn of fatty alcohol acetate
InactiveCN1226270CImprove efficiencyPreparation by ester-hydroxy reactionHeptyl alcoholIsooctyl alcohol
The present invention is the preparation process of fatty alcohol acetate with methyl acetate and fatty alcohol as material. In the presence of catalyst, methyl acetate and fatty alcohol are made to produce ester exchange reaction at the reaction temperature of 70-180 deg.c and the reaction pressure of 0.15-2.0 MPa for 2-6 hr before the reaction material is rectified and separated to obtain product fatty alcohol acetate. The fatty alcohol may one of ethanol, propyl alcohol, isopropyl alcohol, butyl alcohol, isobutyl alcohol, amyl alcohol, isoamyl alcohol, hexyl alcohol, isohexyl alcohol, heptyl alcohol, isoheptyl alcohol, capryl alcohol and isocapryl alcohol. The present invention solves the technological problem that methyl acetate has boiling point lower than that of other matter in the ester exchange system and thus can not produce ester exchange reaction with other fatty alcohol.
Owner:SINOPEC SHANGHAI PETROCHEMICAL CO LTD
Curvularia crescens b-36 and its application in the synthesis of chiral alcohols
ActiveCN111925949BHigh stereoselectivityHigh yieldFungiMicroorganism based processesHeptyl alcoholPolymer science
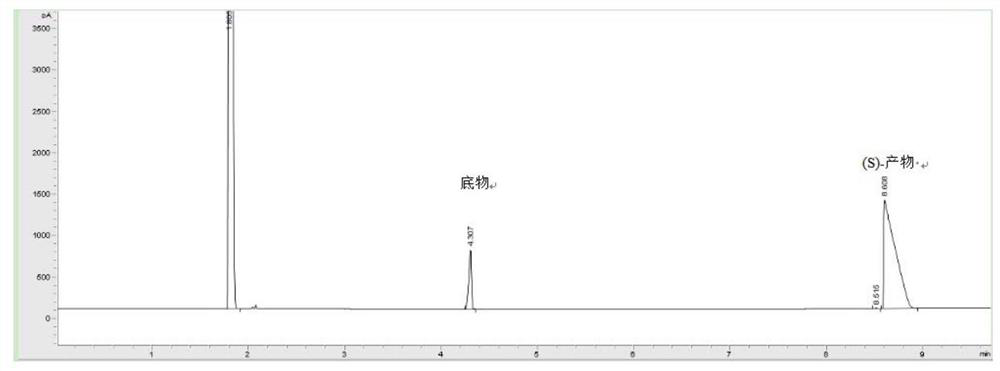
The invention discloses Curvularia hominis B-36 and its application in synthesizing chiral alcohol. The Curvularia crescenae strain B-36 was deposited in the China Center for Type Culture Collection on November 03, 2017, and the preservation number is CCTCC NO: M2017654. The present invention finds for the first time that Curvularia crescens B-36 has the ability to catalyze the reduction of 1-chloro-2-heptanone, and can biotransform 1-chloro-2-heptanone into (S)-1-chloro-2-heptanone Alcohol, simple reaction, high product yield.
Owner:ZHEJIANG MEDICAL COLLEGE
Low-temperature cosolvent of polyoxymethylene dimethyl ether and diesel oil mixed fuel and preparation method of low-temperature cosolvent
PendingCN111253986AStrong targetingImprove solubilityLiquid carbonaceous fuelsFuel additivesHeptyl alcoholCarvacryl acetate
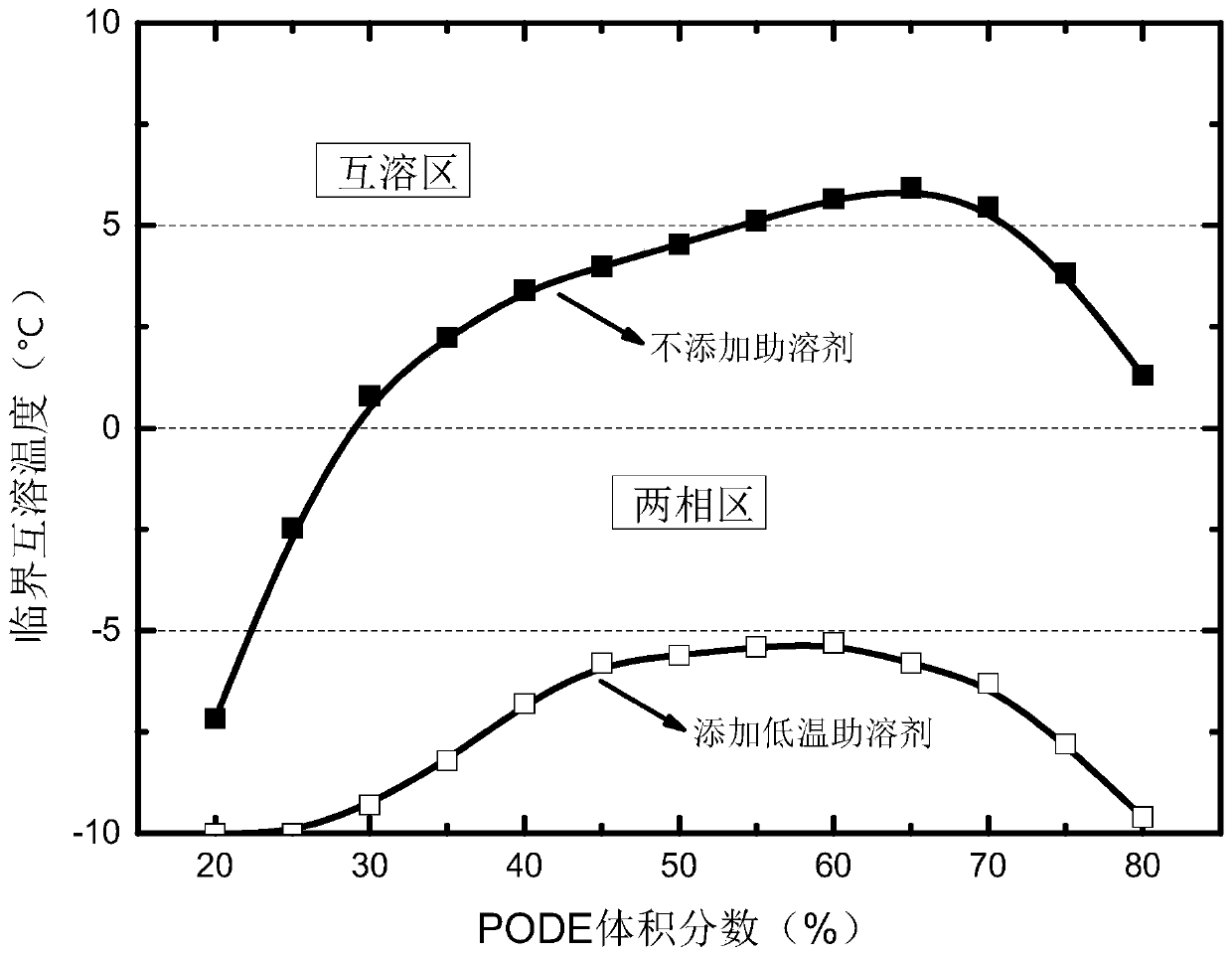
Provided is a low-temperature cosolvent of a polyoxymethylene dimethyl ether and diesel oil mixed fuel and a preparation method of the low-temperature cosolvent, wherein the cosolvent comprises the components in parts by volume: 10 to 20 parts of castor oil, 5 to 10 parts of n-amyl alcohol, 10 to 15 parts of n-hexyl alcohol, 15 to 25 parts of n-heptanol, 25 to 45 parts of n-octyl alcohol and 5 to15 parts of an ethylene-vinyl acetate copolymer. The preparation method comprises the steps: adding n-amyl alcohol into castor oil at room temperature to form a mixed solution, adding n-hexyl alcohol,n-heptanol and n-octanol into the mixed solution in the stirring process, and uniformly mixing, and gradually adding the ethylene-vinyl acetate copolymer in batches in the adding process. According to the low-temperature cosolvent, the mixed fuel can be mutually dissolved in any proportion in low-temperature environments such as high and cold environments and winter environments and can stably coexist for a long time.
Owner:XI AN JIAOTONG UNIV
Cladosporium p-1 and its application in aging of base wine
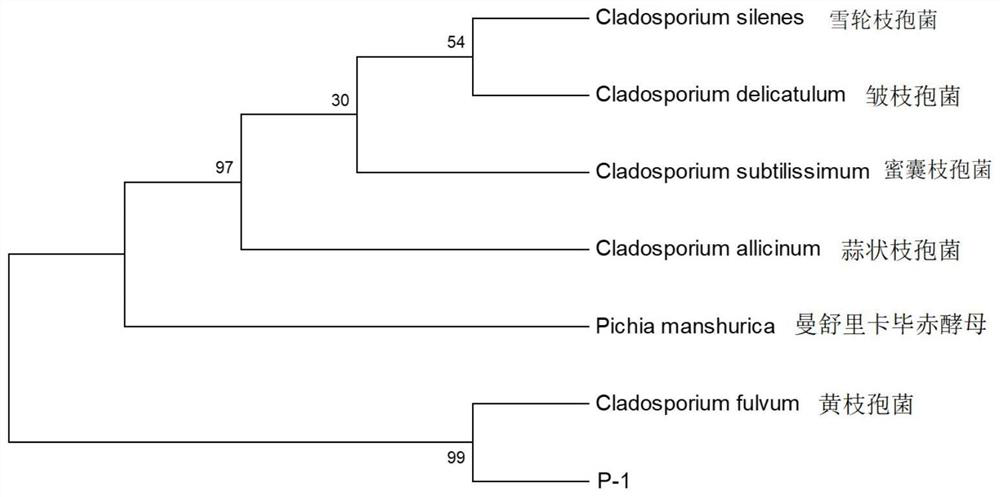
ActiveCN111205989BAccelerated agingSolve the problem of long aging timeFungiAlcoholic beverage preparationHeptyl alcoholPhenethyl alcohol
The invention belongs to the field of food biotechnology, and in particular relates to a Cladosporium P-1 and its application in the aging of base wine. Aiming at the problem that there are no microorganisms that can be used in the aging process of base wine in the existing market, the present invention provides a Cladosporium P‑1 and its application in the aging process of base wine. The preservation number of the Cladosporium P‑1 is CGMCC No.18124, and the ITS sequence is shown in SEQ ID NO:1. Cladosporium P-1 of the present invention can degrade β-phenylethanol, hexanol, nonanol, 1-decanol, 3-methyl-2-octanol or heptanol, and can be used in the aging of base wine to accelerate aging It is of great significance to reduce the relative content of new wine aldehydes, increase the relative content of esters in wine, and improve the quality of product flavor.
Owner:LUZHOU LAOJIAO CO LTD +2
Low-toxicity plasticizer
The invention provides a low-toxic plasticizer, and relates to the technical field of additives. The low-toxicity plasticizer is prepared from the following components: 20 to 35 parts of phthalic anhydride, 12 to 26 parts of 2-propylheptanol, 8 to 16 parts of filler, 3 to 10 parts of a thickening agent, 11 to 18 parts of triethylhexyl phosphoric acid, 1 to 5 parts of methyl amyl alcohol, 4 to 9 parts of a cellulose derivative, 5 to 13 parts of guar gum, 14 to 23 parts of sodium polyacrylate and 2 to 8 parts of plant extractant, wherein the filler comprises ripple filler, grid filler, pall ring filler and cascade ring filler; the mass ratio of the ripple filler to the grid filler to the pall ring filler to the cascade ring filler is (3 to 11) : (2.5 to 5.5) : (2 to 9) : (4.7 to 12.6). The low-toxicity plasticizer provided by the invention has the beneficial effects that the plasticizing effect is good and the environment-friendly effect is good; the plant extract is added into the components of the plasticizer, so that the flexibility of the plasticizer can be improved, and the plasticizer meets green, organic and pure natural idea.
Owner:佛山市高明区生产力促进中心
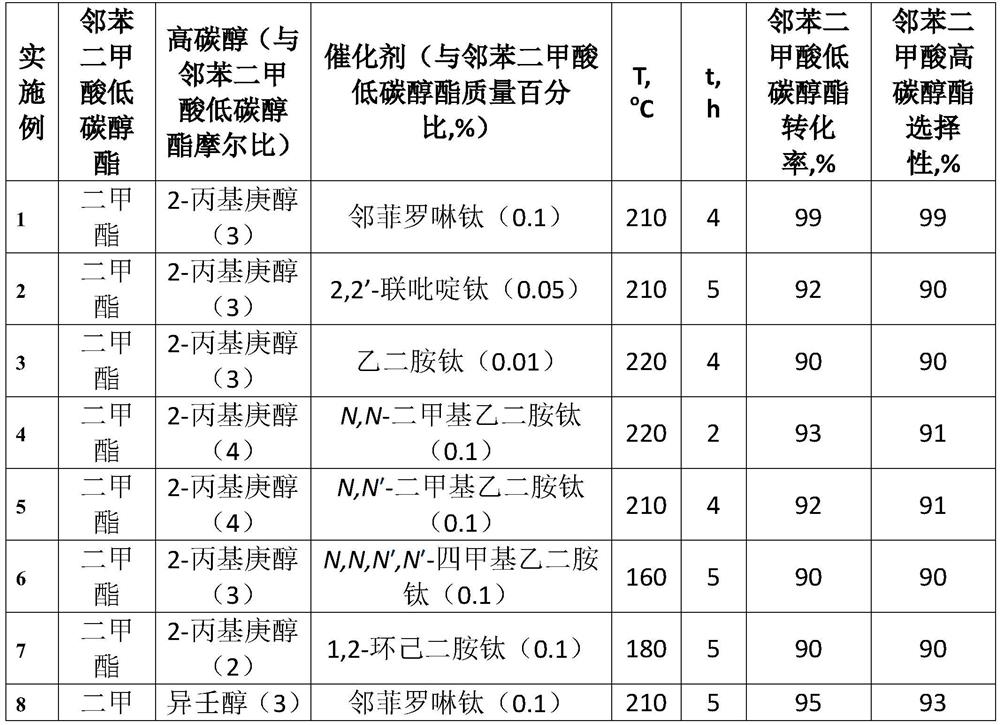
A kind of method that titanium chelate catalyzes transesterification to prepare higher carbon alcohol phthalate
ActiveCN108976116BGood storage and transportation stabilityEasy feedingPreparation by ester-hydroxy reactionHeptyl alcoholPtru catalyst
The present invention relates to a kind of method that titanium chelate catalyzes transesterification to prepare higher carbon alcohol ester of phthalate, with dimethyl phthalate, diethyl phthalate and dibutyl phthalate Low-carbon alcohol phthalate and high-carbon alcohols such as isononanol, isodecyl alcohol, and 2-propylheptanol are used as raw materials, with o-phenanthroline, 2,2'-bipyridine, ethylenediamine, N,N ‑Dimethylethylenediamine, N,N′‑dimethylethylenediamine, N,N,N′,N′‑tetramethylethylenediamine, 1,2‑cyclohexanediamine, etc. The titanium chelate of the ligand is the catalyst, the amount of the catalyst is 0.01-0.1% of the mass of the lower alcohol phthalate, the molar ratio of the higher alcohol to the lower alcohol phthalate is 2-4, and the reaction temperature is 160- 220°C, the reaction time is 2 to 5 hours, the conversion rate of lower alcohol phthalate and the selectivity of higher alcohol phthalate are up to 99%. The invention has the advantages of good raw material storage and transportation stability, convenient feeding, less side reactions, low raw material consumption, no corrosion of the catalyst, and less consumption.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Pretreatment solution for electroless plating and electroless plating method
ActiveUS20170067164A1Chemical resistanceMaintenance conditionLiquid/solution decomposition chemical coatingHeptyl alcoholAlcohol sugars
The pretreatment solution for electroless plating of the present invention is composed of noble metal colloidal nanoparticles, a sugar alcohol, and water. The colloidal nanoparticles are gold, platinum, or palladium, have an average particle diameter of 5 to 80 nm, and are contained in the pretreatment solution in an amount of 0.01 to 10 g / L as metal mass. The sugar alcohol is at least one selected from the group consisting of tritol, tetritol, pentitol, hexitol, heptitol, octitol, inositol, quercitol, or pentaerythritol and is contained in the pretreatment solution in an amount of 0.01 to 200 g / L in total. The electroless plating method of the present invention uses the pretreatment solution and performs the electroless plating in an electroless plating bath.
Owner:EEJA LTD
Pomegranate essence and preparation method thereof
ActiveCN104286811ASimple processReasonable formulaFood ingredient as taste affecting agentFood preparationBiotechnologyHeptyl alcohol
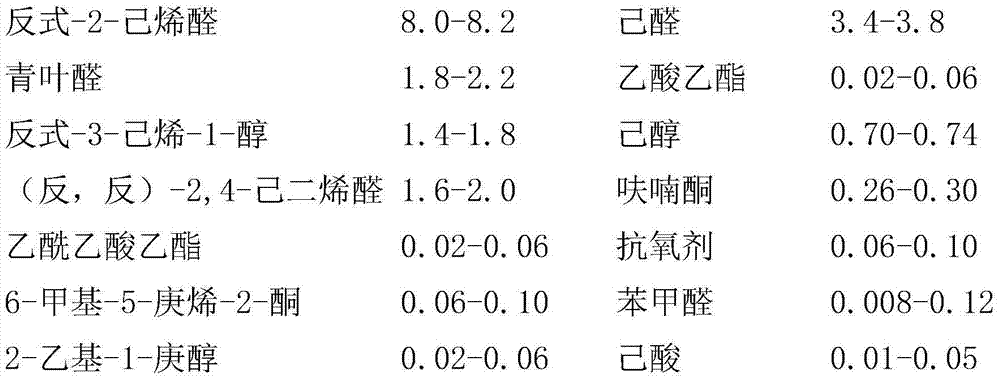
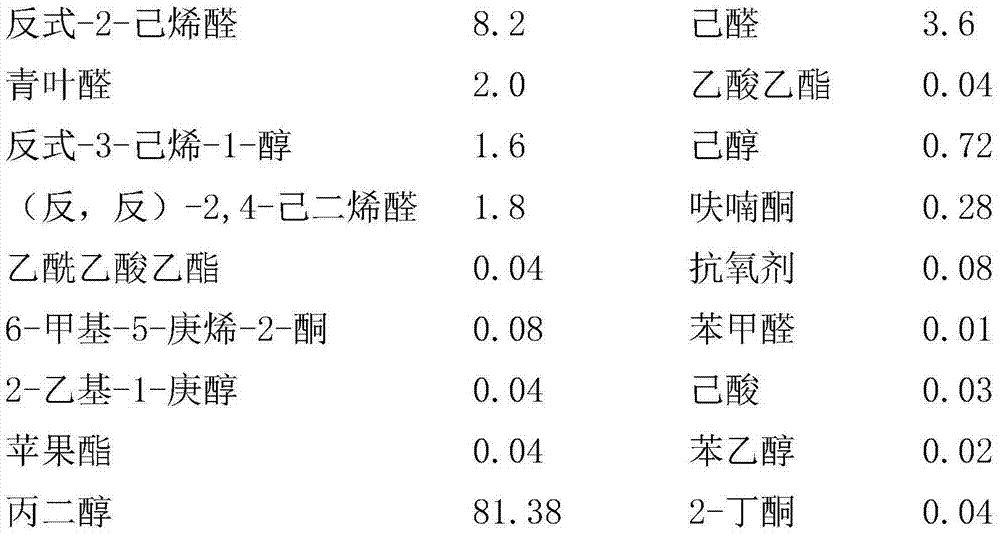
Relating to the technical field of essence preparation, the invention provides a pomegranate essence and a preparation method thereof. The pomegranate essence is composed of the following raw materials by weight: 8.0-8.2 of trans-2-2-hexenal, 3.4-3.8 of hexanal, 1.8-2.2 of leaf aldehyde, 0.02-0.06 of ethyl acetate, 1.4-1.8 of trans-3-hexene-1-ol, 0.70-0.74 of hexanol, 1.6-2.0 of (trans, trans)-2, 4-hexadienal, 0.26-0.30 of furanone, 0.02-0.06 of ethyl acetoacetate, 0.06-0.10 of an antioxidant, 0.06-0.10 of 6-methyl-5-heptene-2-one, 0.008-0.12 of benzaldehyde, 0.02-0.06 of 2-ethyl-1-heptanol, 0.01-0.05 of caproic acid, 0.02-0.06 of fructone, 0.01-0.03 of phenethyl alcohol, 81.36-81.40 of propylene glycol, and 0.02-0.06 of 2-butanone. According to the invention, the prepared pomegranate essence has the characteristics of simple process and reasonable formula, and aims to fill in the blank of pomegranate essence, especially the blank of pomegranate essence with strong natural sense, enhance the natural flavor of pomegranate juice, jam, jelly, fruit wine and other products, and enrich beverage and other daily consumer goods markets.
Owner:ANHUI CHINAHERB FLAVORS & FRAGRANCES
Composition for odour improvement
The invention relates to a preparation containing: (i) a composition containing (a) one, two or a plurality of compounds selected from the group consisting of (a1) alcohol monoterpenes of formula (I) in which R1 is H or methyl, R2 is H or C2-alkenyl, und R3 is a linear or branched, saturated or unsaturated hydrocarbon radical with 4 to 10 carbon atoms, and the enantiomers, diastereomers, racemates, solvates and physiologically compatible salts thereof, and / or (a2) bicyclic epoxy-monoterpenes, (b) at least two lactones of formula (II) in which R4 is H or methyl, R5 is a linear or branched, saturated or unsaturated hydrocarbon radical with 2 to 10 hydrocarbon atoms and n is the number 1 or 2, and the enantiomers, diastereomers and racemates thereof, (c) one, two or a plurality of solvents selected from the group consisting of ethanol, water, dipropylene glycol (DPG), diethyl phtalate (DEP), propylene glycol (PG), isopropyl myristate (IPM), isopropyl palmitate (IPP), triethyl citrate (TEC), triacetin (TRI), 1,2-Propanediol, 1,3-Propanediol, Propanethiol, Pentanediol, Hexanediol, Octanediol, Decanediol (SymClariol®), Dodecanol, 4-hydroxy-acetophenone (SymSave® H), glycerine, butylene glycol, pentylene glycol, hexylene glycol, decylene glycol, propylene carbonate, butylene carbonate, glycerine carbonate, 2-5 benzyl heptanol, lauryl alcohol, trimethyl-hydroxypentyl-isobutyrate, glyceryl-caprylate, ethylhexyl glycerine, benzyl benzoate (BB), and optionally (d) other flavouring agents or aromatic substances selected from the group consisting of 3-phenylbutanal (Trifernal), acetyl methyl carbinol, anethole, anisyl acetate, dihydroeugenol, linalyl formate, 2-methyldecanal, 2-benzyl-2-methylbut-3-ene nitrile (Ci-Trowanil® B), 3-hexenyl acetate, styrallyl acetate, belanis, citronellal, cinnamyl acetate, rhubafuran, beta-ions, anther, prenyl acetate, 2-phenyl propanal, 4-(4-hydroxyphenyl)butan-2-one (Frambinon®), ethyl phenoxyacetate, isoralderine, gamma-terpinene, limonene, neocyclocitral, methyl lavender ketone, styrallyl propionate, phenyl ethyl propionate, limonenal, 4-isopentylcyclohexanol (Symrose®), 4-methyl-2-phenyl-3,6-dihydro-2H-pyran / 4-methylene-2-phenyl-tetrahydropyrane (Rosyrane super), hydrocitronitril, phenoxanol, isoamyl phenylacetate, damascone, silvial, nectaryl, ambroxide, acetyl pyrazine, trimethyl pyrazine, isoamyl acetate, para-cresyl methyl ether, filbertone, cyclohexyl acetate, heliotropin, acetophenone, anisaldehyde, para-methyl acetophenone, veratraldehyde, methyl anisate and vertoprenal; (ii) aldehydes of formula (III) in which R6 is a saturated or non-saturated, linear hydrocarbon radical; and / or (iii) free fatty acids of formula (IV), in which R7 is a linear or branched, saturated hydrocarbon radical.
Owner:SYMRISE GMBH & CO KG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com