Ester plasticizers based on ethylhexanol and propylheptanol
A kind of technology of ethyl hexanol and propyl heptanol, applied in the field of thermoplastic composition, can solve the problems such as infrequent use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Ester Production
[0068] The amounts of 2-ethylhexanol ("2EH") and 2-propylheptanol ("2PH") shown in Table 1 below were compared under nitrogen with trimellitic anhydride ("TMA"; see table for respective amounts) and 500 ppm tin-(II)-oxalate (Evonic) heating, said amount is based on the total amount of the composition. The 2-propylheptanol used was of technical grade purity and thus consisted of 80% 2-propylheptanol and 20% decanol isomers other than 2-propylheptanol. The esterification reaction starts at about 150°C, water is formed, and the temperature rises slowly to 220°C. After 4 hours, the water of reaction was further removed and the vacuum was reduced to about 5 mbar over 10 hours. The reaction was stopped when the acid number dropped below 1. The remaining unreacted alcohol was removed by distillation. After this time, the product was filtered.
[0069] Table 1: Amounts of Compounds Used
[0070] Example
Embodiment 2
[0071] Embodiment 2: the mensuration of the physical property of ester
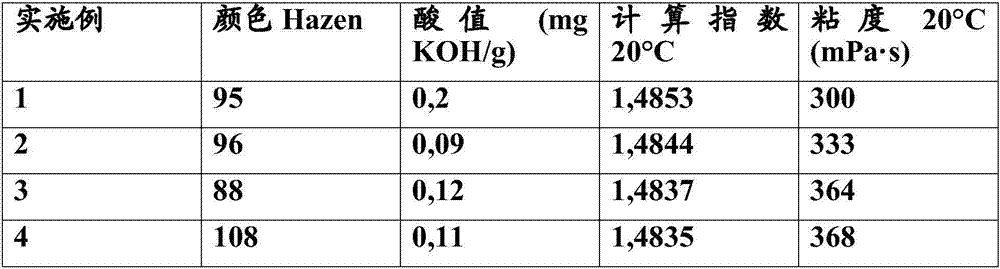
[0072] The degree of discoloration of esters is measured in Hazen units following the method described in DIN EN ISO 6271-2005.
[0073] The acid number is determined according to DIN 53402.
[0074] Viscosity was measured using DIN 53019.
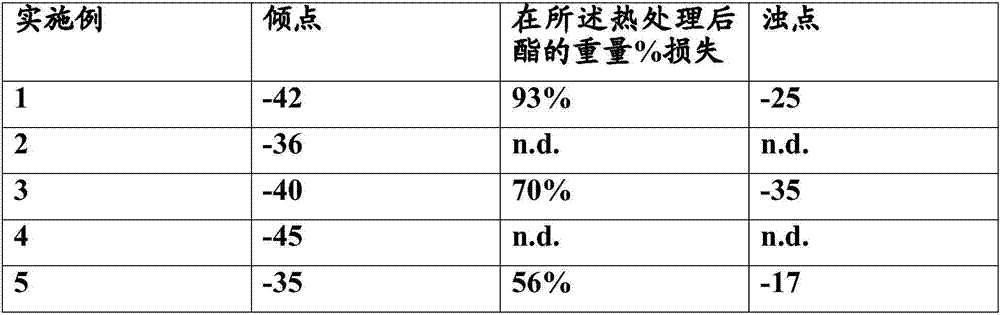
[0075] Furthermore, the pour point is measured according to DIN ISO 3016 (10 / 82), which indicates the lowest temperature at which a liquid becomes semisolid and loses its flow properties.
[0076] The cloud point is determined according to DIN EU 23015.
[0077] Using a Netzsch instrument for thermogravimetric analysis, the sample was heated at a rate of 10 K / min at N 2 The volatility of the ester was tested by heating from 20°C to a final temperature of 300°C. After the sample reached a temperature of 300° C., the sample was kept at this temperature for 30 minutes.
[0078] The results are shown in Tables 2 and 3 below:
[0079] Table 2:
[0080]
[0081] ...
Embodiment 3
[0085] Example 3: Thermoplastic Compositions Comprising Esters
[0086] Prepare the following compositions:
[0087]
[0088] Table 4: Preparation of test specimens
[0089] The components indicated above were mixed together and milled on a laboratory mill at 185°C for 5 minutes. The Shore A hardness of these specimens was tested according to DIN ISO 868 (three measurements per sample) using rolled plates, 3 mm pressed plates were manufactured in a laboratory press at 180°C. The results are shown in Table 5 below:
[0090]
[0091] Examples B8 and B9 are according to the invention.
[0092] As can be seen from the above examples, the optimized esters of the present invention can be used as plasticizers in the processing of thermoplastic resins. The ester shows good compatibility with polymers, low volatility, and also provides excellent low temperature performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com