Curvularia crescens b-36 and its application in the synthesis of chiral alcohols
A technology of B-36, Curvularia, applied in microorganism-based methods, fungi, microorganisms, etc., can solve the problem of limited number of strains, and achieve the effect of high yield and high stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Optimized Medium for Curvularia hominis B-36 Strain
[0043] Through response surface experiment design, the optimized medium composition of Curvularia hominis B-36 strain was obtained: glucose 15-30g / L, peptone 20-35g / L, K 2 HPO 4 1.0~4.0g / L, (NH 4 ) 2 SO 4 2.5~6.0g / L, NaCl1.0~2.5g / L. The culture conditions of Curvularia hominis B-36 are: initial pH 4.0-7.0, conical flask liquid volume 100-200mL / 500mL conical flask, culture temperature 20-40℃, shaker speed 180-240rpm, inoculum volume 1-10% , Culture time 12~36h.
[0044] Screening of Curvularia hominis B-36 strain:
[0045] (1) Enrichment culture: Inoculate the soil samples collected from different sampling points in Shangyu District, Shaoxing City, into the enrichment medium, and culture it on a shaker at 30°C and 200rpm for 7 days. After the culture medium becomes turbid, take 1mL for culture The culture medium was transferred to fresh enrichment medium, and cultured for 7 days, and thus circulated for 3 tim...
Embodiment 2
[0057] Biocatalytic Reaction: Catalytic Reduction of 1-Chloro-2-Heptanone to Prepare (S)-1-Chloro-2-Heptanol
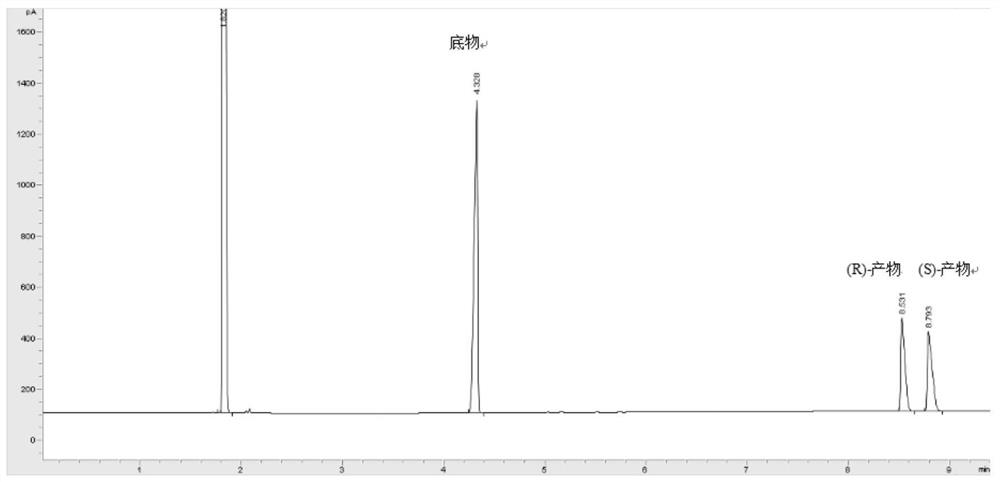
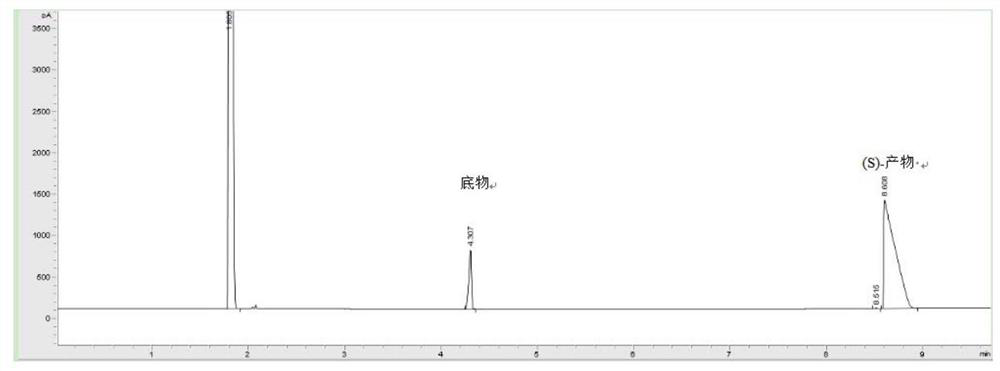
[0058] The wet thalline cell of embodiment 1 gained is suspended in 20mL phosphate buffer saline K 2 HPO 4 -KH 2 PO 4 (pH 6.0), the wet bacterial cell concentration is 40g / L by dry weight; add 50mM 1-chloro-2-heptanone as a substrate, and then add 50g / L glucose as an auxiliary substrate for coenzyme regeneration , 30°C, 200rpm, reacted for 24h. After the reaction, the reaction solution was centrifuged, and the supernatant was taken, added an equal volume of ethyl acetate for extraction, and the content of (S)-1-chloro-2-heptanol was detected by gas phase.
[0059] Product concentration detection and its separation and purification method:
[0060] (1) Concentration detection: After the biocatalytic reaction is completed, the reaction solution is extracted with an equal volume of ethyl acetate. The unreacted substrate and the product in the extract can be analyze...
Embodiment 3~5
[0071] The cells obtained in Example 1 were suspended in 20 mL of phosphate buffer K with different pH values. 2 HPO 4 -KH 2 PO 4 In this method, the concentration of cells by dry weight is 40g / L; 50mM 1-chloro-2-heptanone is added as a substrate, and 50g / L glucose is added as an auxiliary substrate, and the reaction is carried out at 30°C and 200rpm for 24h. Using the product concentration detection method of Example 2, the concentration and ee value of the product (S)-1-chloro-2-heptanol are shown in the table below. The yields of Examples 3-5 are respectively 85%; 97.8%; 80.6%.
[0072]
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com