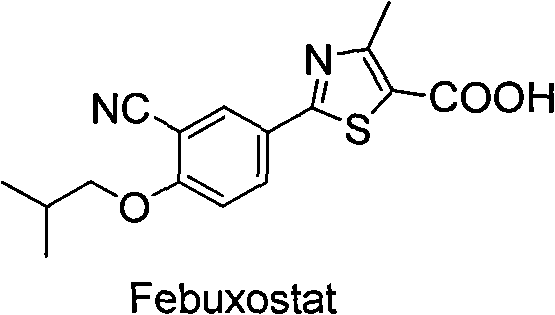
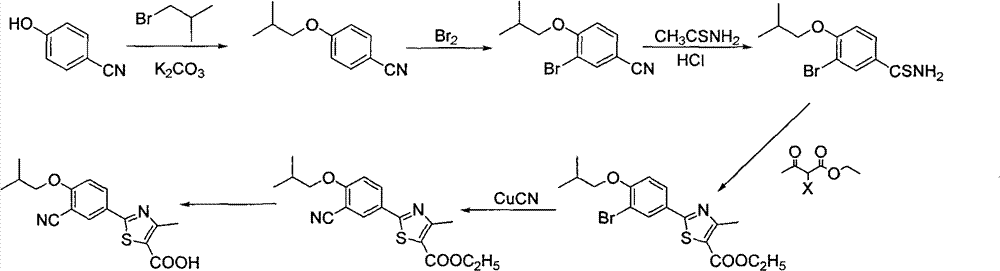
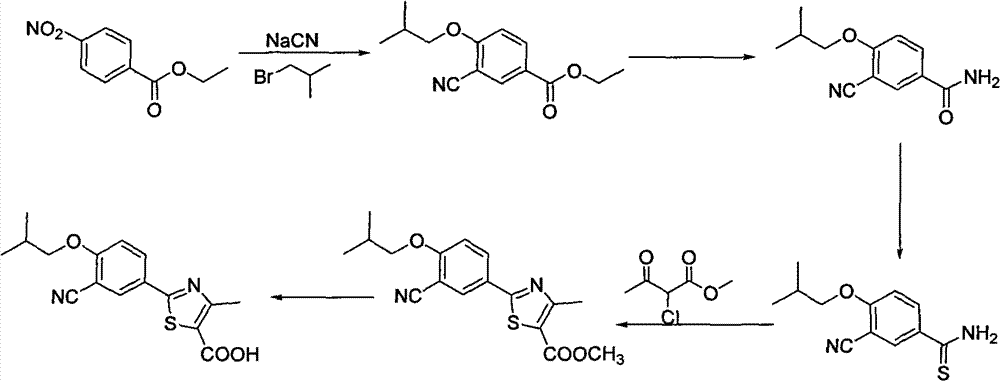
Synthetic method of 2-aryl nitrile thiazole derivatives and intermediates
A compound and bromine technology, applied in the field of heterocyclic chemistry and organic chemistry, can solve the problems of serious equipment corrosion, difficult separation and purification, and difficulty in industrialization, and achieve the effects of reducing reaction costs, simple reaction types, and complete reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of 4-isobutoxybenzonitrile
[0062] Add p-hydroxybenzonitrile (119.0g, 1.0mol) and 400mL N,N-dimethylformamide successively in a 1000ml four-neck round bottom flask, drop into anhydrous potassium carbonate (207.0g, 1.5mol ), sodium iodide (5.0g, 0.03mol) and bromoisobutane (274.0g, 2.0mol), slowly warming up to 80 ~ 85 ℃, stable reaction at this temperature, tracking by TLC during the reaction, until the raw material is completely Transformation takes about 20 hours. After the reaction, filter with suction, rinse the filter cake with 30mL N,N-dimethylformamide, add n-hexane to the obtained solid, extract with 5% aqueous sodium hydroxide solution, combine the organic phases, and concentrate to obtain an orange oil 172.0 g of 4-isobutoxybenzonitrile, the yield was 98.28%, and the HPLC purity was 98.67%.
[0063] 1 H NMR (400 MHz, DMSO) δ 0.99(d, J=5.2 Hz, 6H), 1.99-2.07(m, 1H), 3.83(d, J=5.2 Hz, 2H), 7.09-7.11(m, 2H) , 7.74-7.77(m, 2H). ...
Embodiment 2
[0064] Embodiment 2: the preparation of 4-isobutoxybenzamide
[0065] Add 4-isobutoxybenzonitrile (105.0g, 0.6mol), methanol 600mL and 240.0g 5% NaOH aqueous solution successively in the 2000ml four-necked round bottom flask, heat up to reflux, slowly add 10% hydrogen peroxide 408.0g dropwise under the reflux state, After the dropwise addition was completed, the reaction was continued at this temperature for 8 hours. After the reaction is complete, the reaction system is cooled to 20 to 25° C. by suction filtration, and the filter cake is rinsed with 300.0 g of water, and then dried to obtain 106.5 g of white needle-like crystals 4-isobutoxybenzamide. The yield is 92.00%, HPLC purity 99.87%.
[0066] 1 H NMR (400 MHz, DMSO) δ 0.98(d, J=5.2 Hz, 6H), 1.98-2.06(m, 1H), 3.80(d, J=5.2 Hz, 2H), 6.95-6.98(m, 2H) , 7.1 8(br,1H), 7.82-7.85(m,3H).
Embodiment 3
[0067] Embodiment 3: Preparation of 3-bromo-4-isobutoxybenzamide
[0068] Add 4-isobutoxybenzamide (96.5g, 0.5mol), 600mL dichloroethane and zinc bromide (1.75g, 0.008mol) successively into a 1000ml four-neck round bottom flask, and drop Bromine (88.0 g, 0.55 mol) was added, and after the dropping, the temperature was raised to 65° C. to continue the reaction for 20 hours, and the tail gas was absorbed with 30% NaOH aqueous solution. After the reaction was completed, the reaction was quenched with saturated aqueous sodium bisulfite solution, and the layers were allowed to stand, the organic phase was concentrated, and 250 mL of absolute ethanol was added to the concentrate for recrystallization, and a large amount of white needle-like crystals 3-bromo-4-iso Butoxybenzamide 124.58g, yield 91.60%, HPLC purity 95.71%.
[0069] 1 H NMR (400 MHz, DMSO) δ1.02(d, J=5.2 Hz, 6H), 2.02-2.09(m, 1H), 3.90(d, J=5.2 Hz, 2H), 7.15(d, J=7.2 Hz, 1H), 7.30(br, 1H), 7.86-7.88(m, 1H), 7.93(br,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com