Steroid compound
A technology of steroidal compounds and compounds, applied in the direction of steroidal compounds, drug combinations, organic chemistry, etc., can solve the problems of insufficiency, achieve the effects of less production, low activity, and reduced local side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
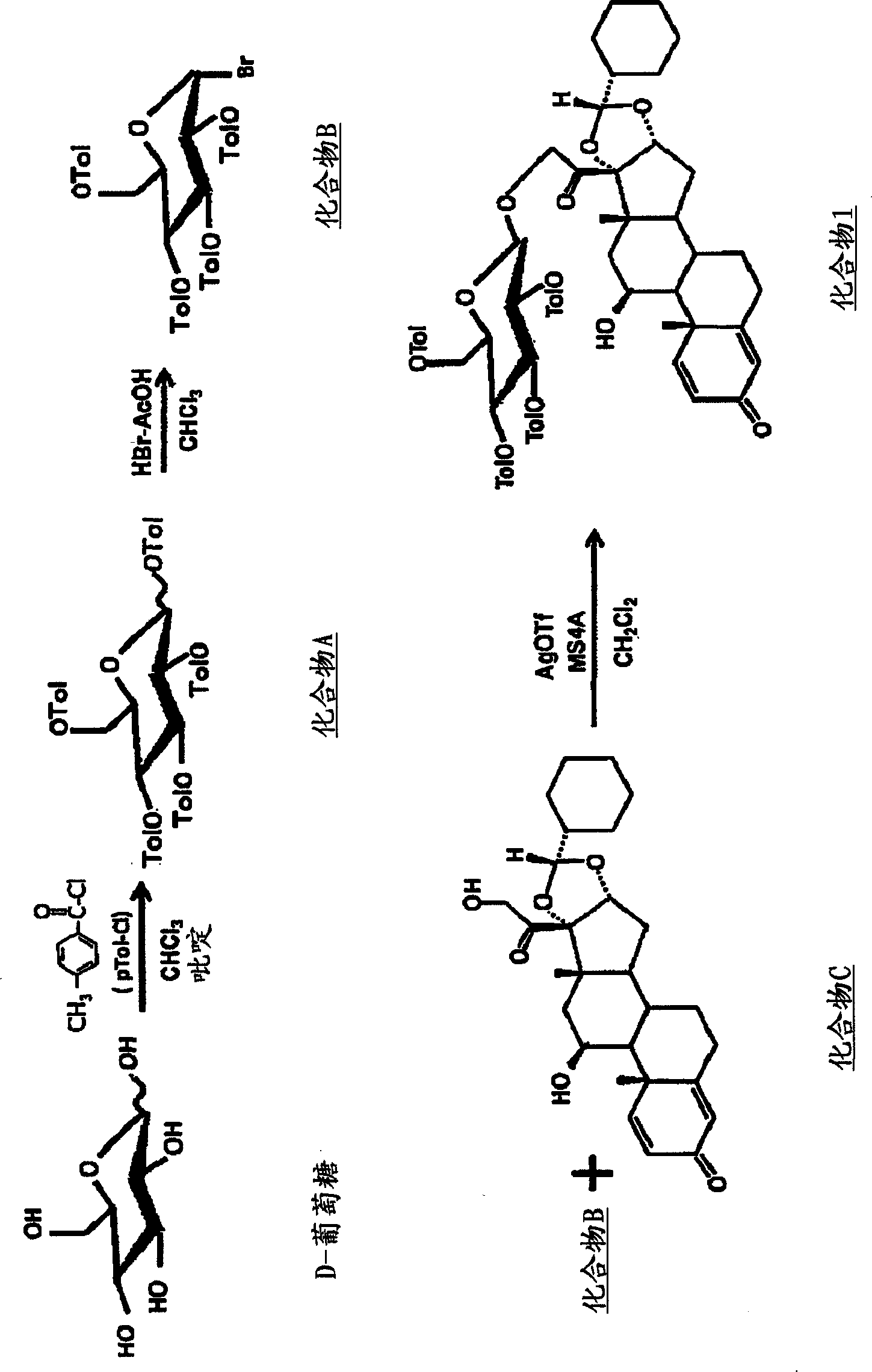
[0042] Synthesis of Compound 1 ( figure 1 )
[0043]Synthesis of compound A: 1.2 g of D-(+)-glucose was dissolved in 40 mL of chloroform, and 14.5 mL of p-toluoyl chloride and 8.9 mL of pyridine were added dropwise at 0-5°C. The mixture was stirred for 6 hours while slowly returning the temperature to room temperature. The reaction solution was poured into ice water, extracted with chloroform, and the organic layer was washed with saturated aqueous copper sulfate, saturated aqueous sodium bicarbonate and saturated brine. After drying over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure. Of the obtained residues, 5.33 g of the residue was isolated and purified by silica gel column chromatography (toluene:ethyl acetate=50:1), to obtain 4.5 g of Compound A as a white powder.
[0044] 1 H-NMR (CDCl 3 )δ: 2.309(3H, s), 2.315(3H, s), 2.362(3H, s), 2.408(3H, s), 2.474(3H, s), 7.101(2H, d, J=8.06), 7.106 (2H, d, J = 8.06), 7.156 (2H, d, J = 8.06...
Embodiment 2
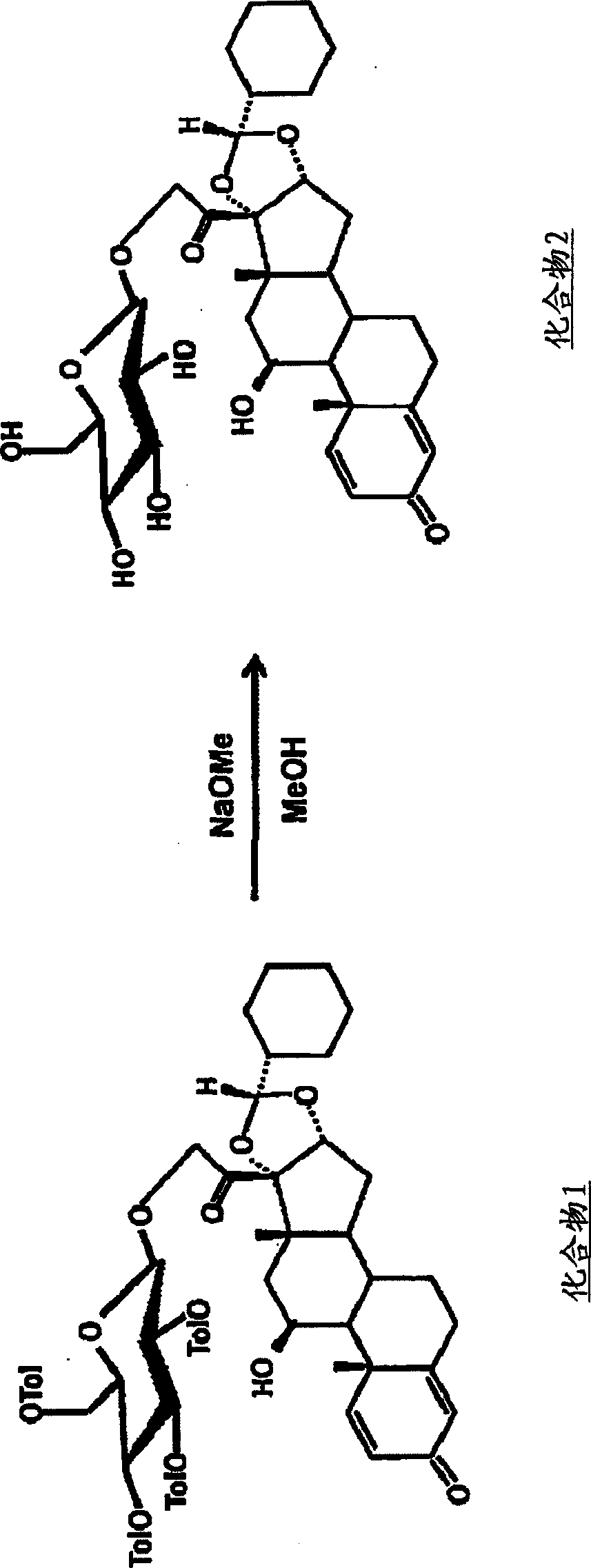
[0054] Synthesis of Compound 2 ( figure 2 )
[0055] Compound 1 (887 mg) was dissolved in anhydrous methanol (10 mL) and anhydrous chloroform (5 mL), and 28% sodium methoxide (1 mL) was added dropwise at room temperature under an argon atmosphere, and the reaction was further stirred for 1 hour. Add strong acidic cation exchange resin Dowex50W (H + ) for neutralization, suction filtration, and after washing with methanol, the filtrate was distilled off under reduced pressure, and the residue was purified by silica gel column chromatography (chloroform:methanol=10:1) to obtain 433mg (85.3%) of Compound 2.
[0056] Rf=0.36 (chloroform:methanol=5:1)
[0057] 1 HNMR (CD 3 OD)δ: 0.94(3H, s), 0.96-1.32(8H, m), 1.49(3H, s), 1.50-1.80(9H, m), 1.80-2.00(2H, m), 2.07-2.30(2H , m), 2.32-2.43(1H, m), 2.57-2.72(1H, m), 3.21-3.40(4H, m), 3.62-3.71(1H, m), 3.84-3.92(1H, m), 4.32 (1H, d, J = 7.8Hz), 4.36 (1H, d, J = 4, 2Hz), 4.54 (1H, d, J = 18.9Hz), 4.76 (1H, d, J = 18.9Hz), 4.84 ( ...
Embodiment 3
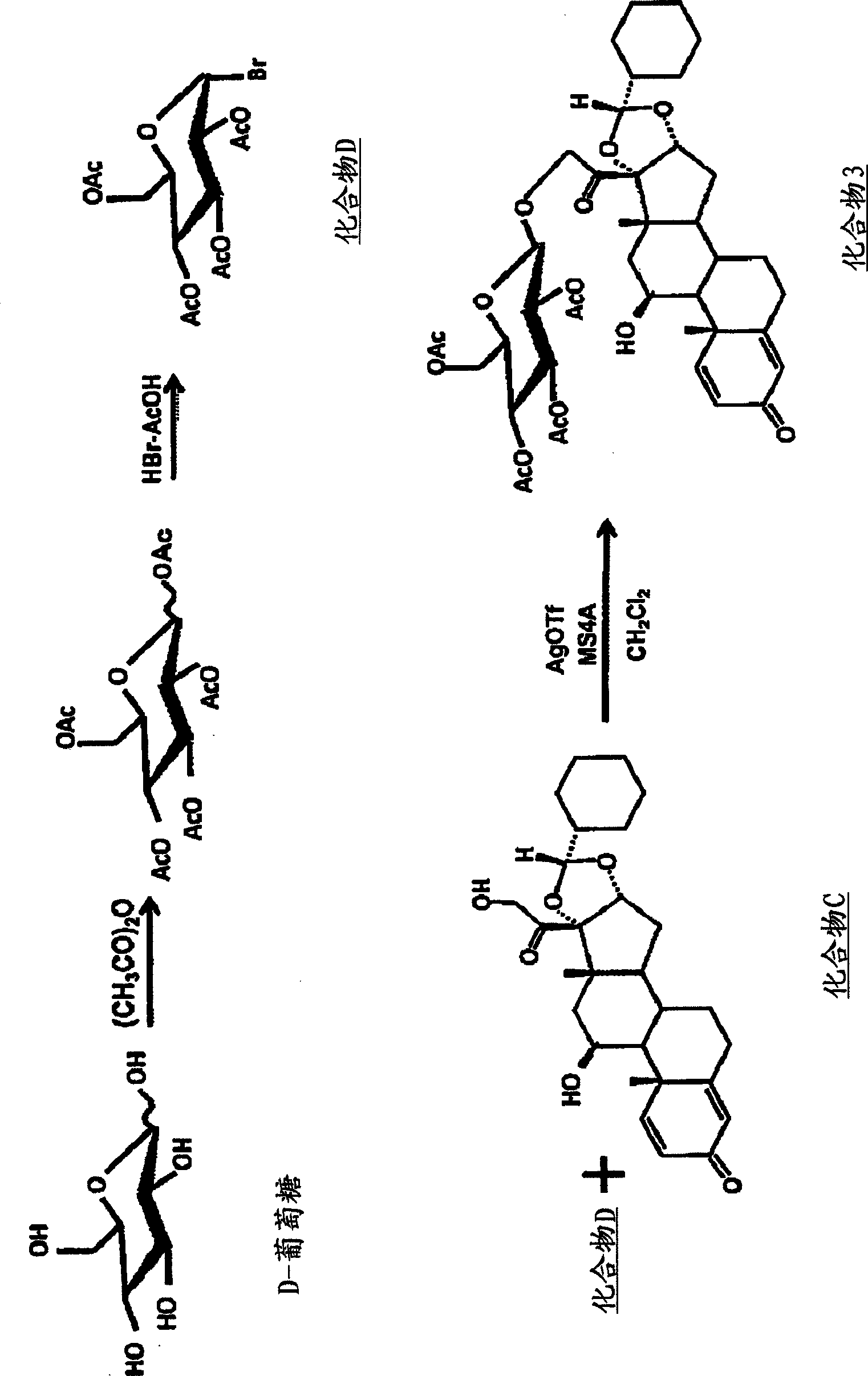
[0059] Synthesis of Compound 3 ( image 3 )
[0060] Synthesis of compound D: D-(+)-glucose (2.02g), acetic anhydride (10mL), 33wt% hydrogen bromide / acetic acid (2mL) were stirred overnight at room temperature and protected from light, and then 33wt% of Hydrogen bromide / acetic acid (10 mL) was stirred for 7 hours. Dichloromethane (50 mL) was added to the reaction liquid, and the mixture was poured into ice water (50 mL) for liquid separation. Extract the aqueous layer with dichloromethane (50mL×2), combine the dichloromethane layers, neutralize with aqueous sodium bicarbonate, wash with water, wash with brine, and dry (MgSO 4 ), and then concentrated under reduced pressure. Diisopropyl ether (6.5 g) was added to the concentrated residue (4.74 g), and compound D synthesized separately was inoculated. The solidified mass was crushed, collected by filtration, washed with diisopropyl ether, and dried under reduced pressure (room temperature) to obtain compound D (3.83 g, 83.1%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com