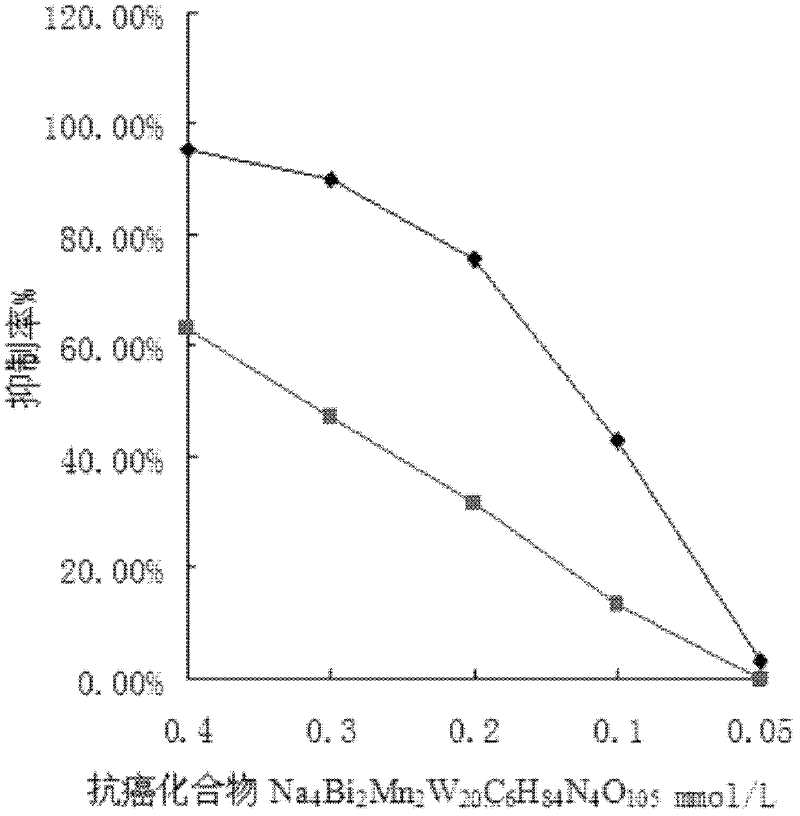
Method for synthesizing anticancer compound Na4Bi2Mn2W20C6H84N4O105
A technology of na4bi2mn2w20c6h84n4o105 and a synthesis method is applied in the field of synthesis of anticancer compounds to achieve the effect of strong proliferation inhibition and good inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0008] Specific embodiment one: the anticancer compound Na of this embodiment 4 Bi 2 mn 2 W 20 C 6 h 84 N 4 o 105 The synthetic method of is realized according to the following steps: one, under magnetic stirring, the Na of 4~5mmol 2 WO 4 Dissolve in 80-120ml of deionized water, and heat to 80-120°C, then add 6mol / L HCl dropwise to adjust the pH to 5.0-7.0 to obtain solution A; 2. Mix 0.5-1mmol of MnCl 2 , 0.8-1.2 mmol of solid imidazole and 0.3-0.8 mmol of Bi(NO) dissolved in 6 mol / L HCl 3 ) 3 At the same time, add it to solution A, mix it and heat it to 80-120°C for 1-2 hours, then cool it to room temperature, filter it and let it rest for 5-10 days to complete the anticancer compound Na 4 Bi 2 mn 2 W 20 C 6 h 84 N 4 o 105 Synthesis; wherein the amount of HCl in step 2 is 10mL.
[0009] The obtained anticancer compound Na in the present embodiment 4 Bi 2 mn 2 W 20 C 6 h 84 N 4 o 105 It is yellow blocky crystal.
[0010] The cooling in the second st...
specific Embodiment approach 2
[0011] Specific implementation mode 2: The difference between this implementation mode and specific implementation mode 1 is that in step 1, 4~? mmol Na 2 WO 4 Dissolve in 80ml of deionized water and heat to 80°C. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0012] Specific embodiment three: the difference between this embodiment and specific embodiment one is that in step one, 5 mmol of Na 2 WO 4 Dissolve in 120ml of deionized water and heat to 120°C. Other steps and parameters are the same as those in Embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com