Human fibrinogen-like protein 2 prothrombinase immunogenicity peptide and use thereof
A fibrin, immunogenic technology, applied in the direction of anti-enzyme immunoglobulin, peptide, antibody, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Synthesis of Example 1 Antigen
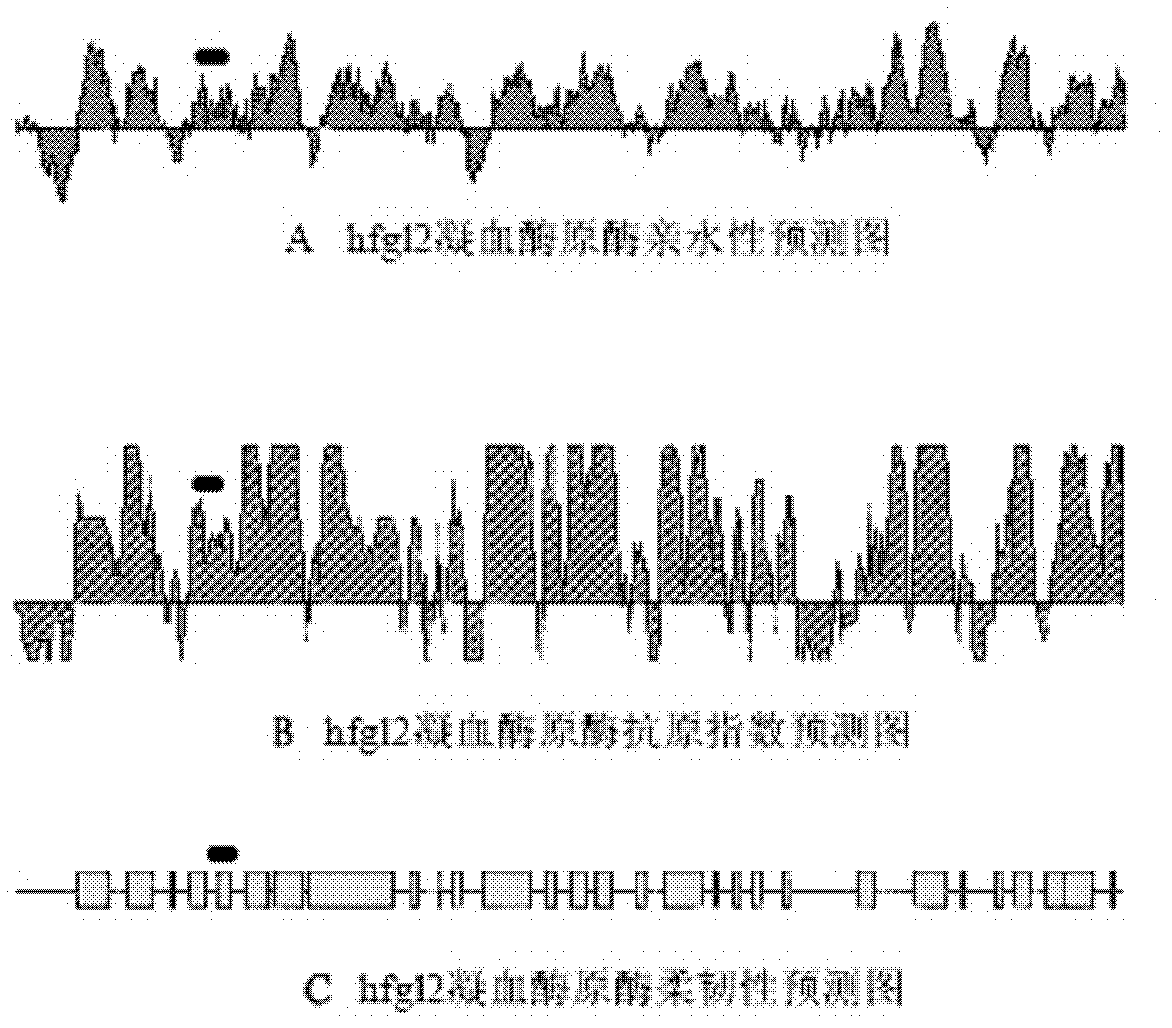
[0038] Using DNASTAR and HomoloGene software, according to the hydrophilicity, antigenicity, flexibility and exposure of hfgl2 prothrombinase extracellular N-terminal amino acid, using the Jameson-Wolf algorithm, through the computer antigen index prediction analysis, select the hydrophilicity, antigenicity NPG-12, a peptide with 12 consecutive amino acids with high index, good flexibility, rich in glutamic acid residues, exposed on the surface of the protein molecule, without homology within the species, and near the 91st serine residue The amino acid sequence is: Glu-Glu-Val-Phe-Lys-Glu-Val-Gln-Asn-Leu-Lys-Glu. According to its amino acid sequence, PSSM-8 automatic peptide synthesizer was used to synthesize the peptide by FOMC method. NPG-12-BSA complete antigen was synthesized by glutaraldehyde coupling method with BSA as carrier protein. After the antigen synthesis was completed, it was stored in a -70°C refrigerator for later use....
Embodiment 2A
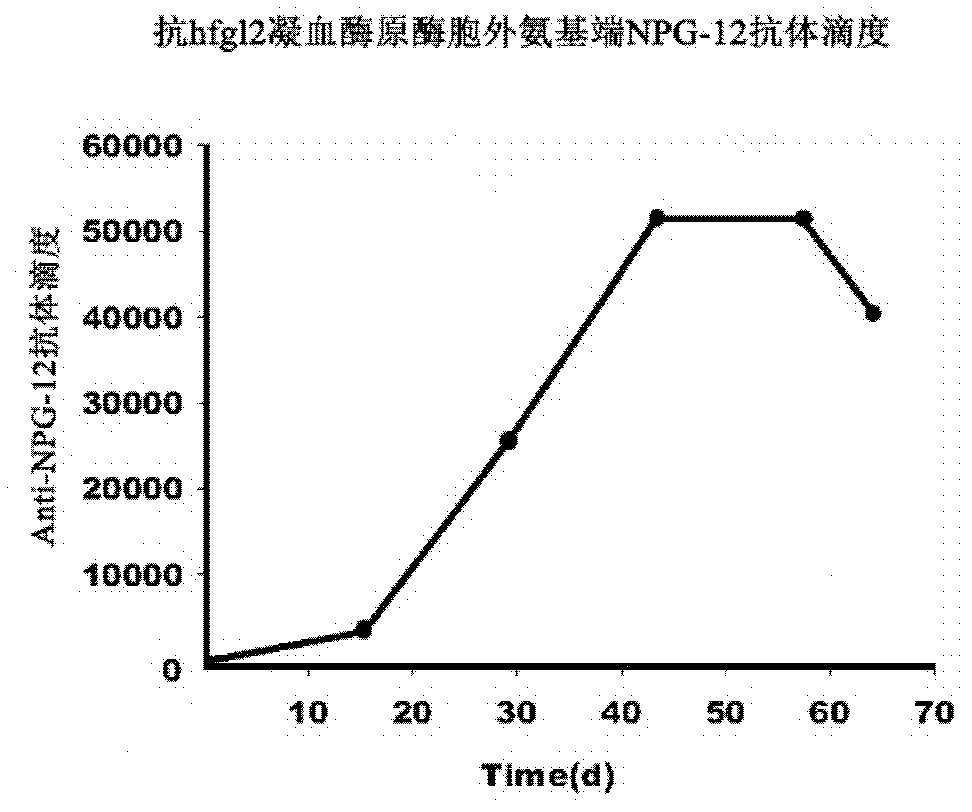
[0039] Embodiment 2 Preparation of Anti-NPG-12 antibody
[0040] (1) Animal immunity
[0041] Anti-NPG-12 antibody was prepared by immunizing male New Zealand white rabbits (clean grade, weighing 2.5-3 kg) with the prepared antigen together with complete or incomplete Freund's adjuvant. Set up the immunization group and the pseudo-immunization group. The immunization group: take 500 μg of antigen per rabbit for the initial immunization, and 250 μg of antigen per rabbit for subsequent immunization, and add an equal volume of Freund’s adjuvant to both. Incomplete Freund's adjuvant, fully emulsified, injected subcutaneously on the back and hind legs of rabbits. Booster immunization every two weeks, a total of 5 times. Sham immunization group: Sham immunization with normal saline plus Freund's adjuvant, the dose and method were the same as the immunization group.
[0042] (2) Collection of serum samples
[0043] From the first immunization, 1 ml of blood was drawn from the vei...
Embodiment 3
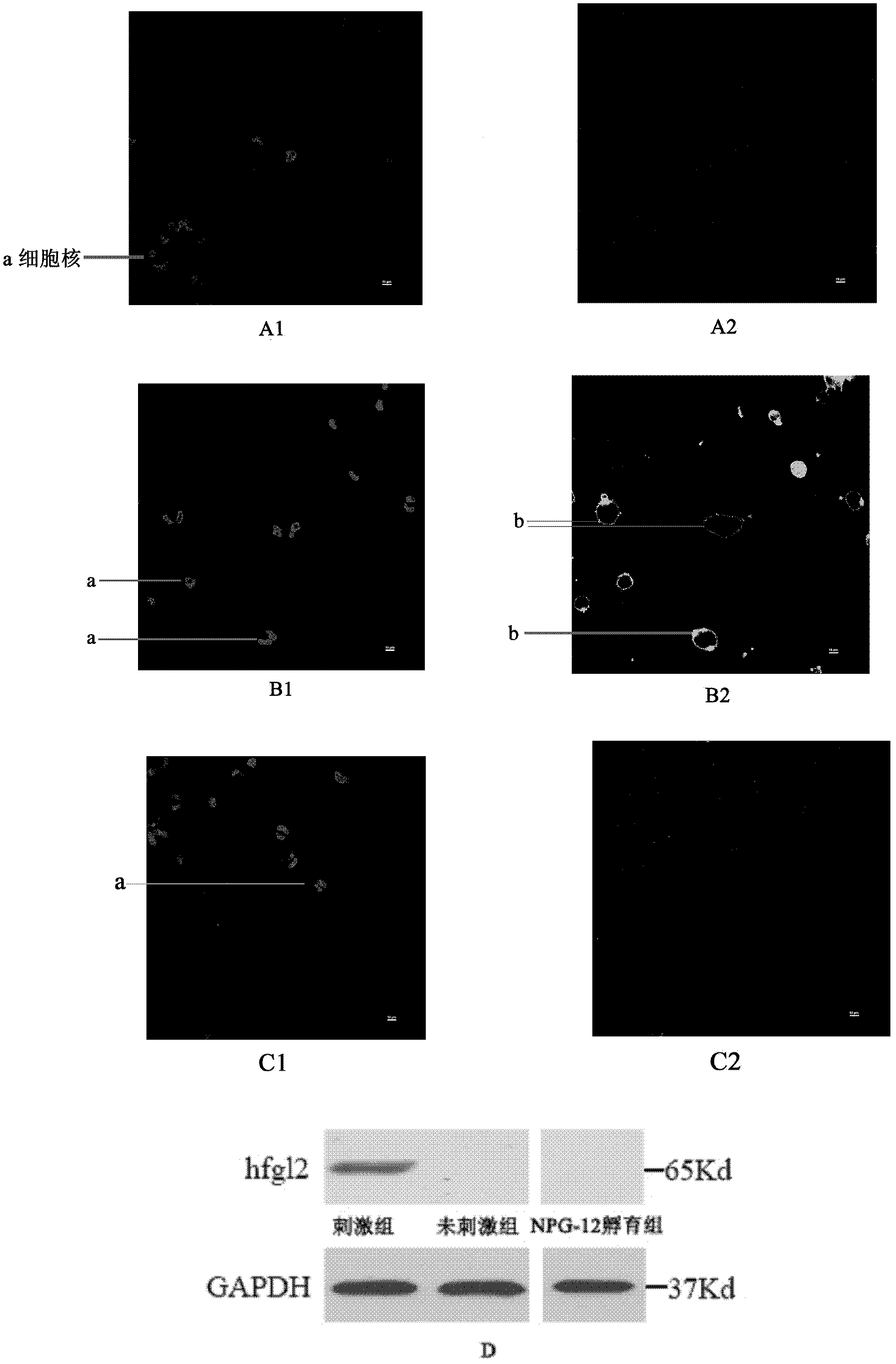
[0046] Example 3 Establishment of cell model expressing hfgl2 prothrombinase
[0047] Human acute monocytic leukemia cell line THP-1 cells (purchased from American Standard Biological Collection) were stimulated with 20ng / ml IFN-γ for 12 hours, and the cells could express hfgl2 prothrombinase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com