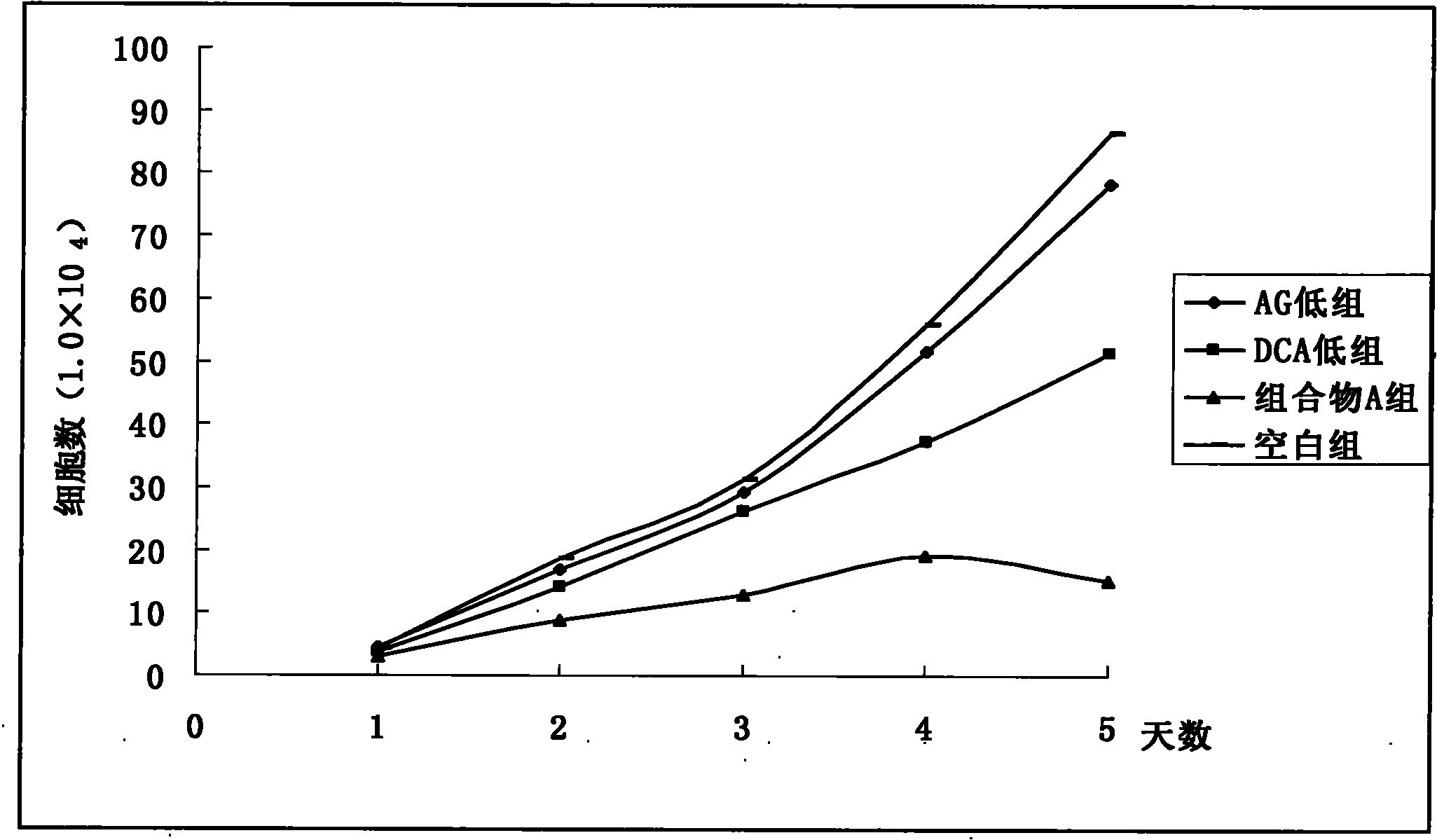
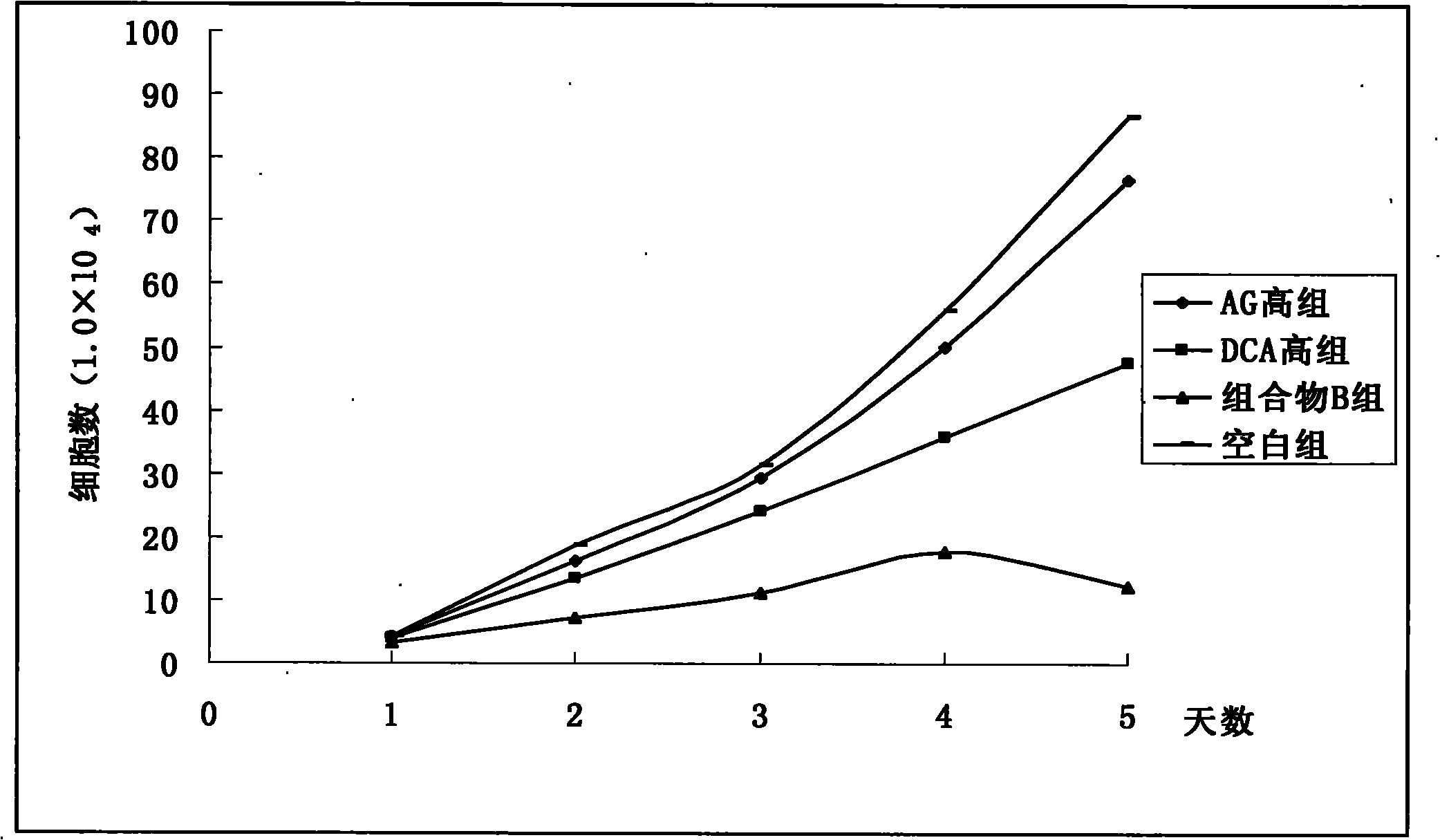
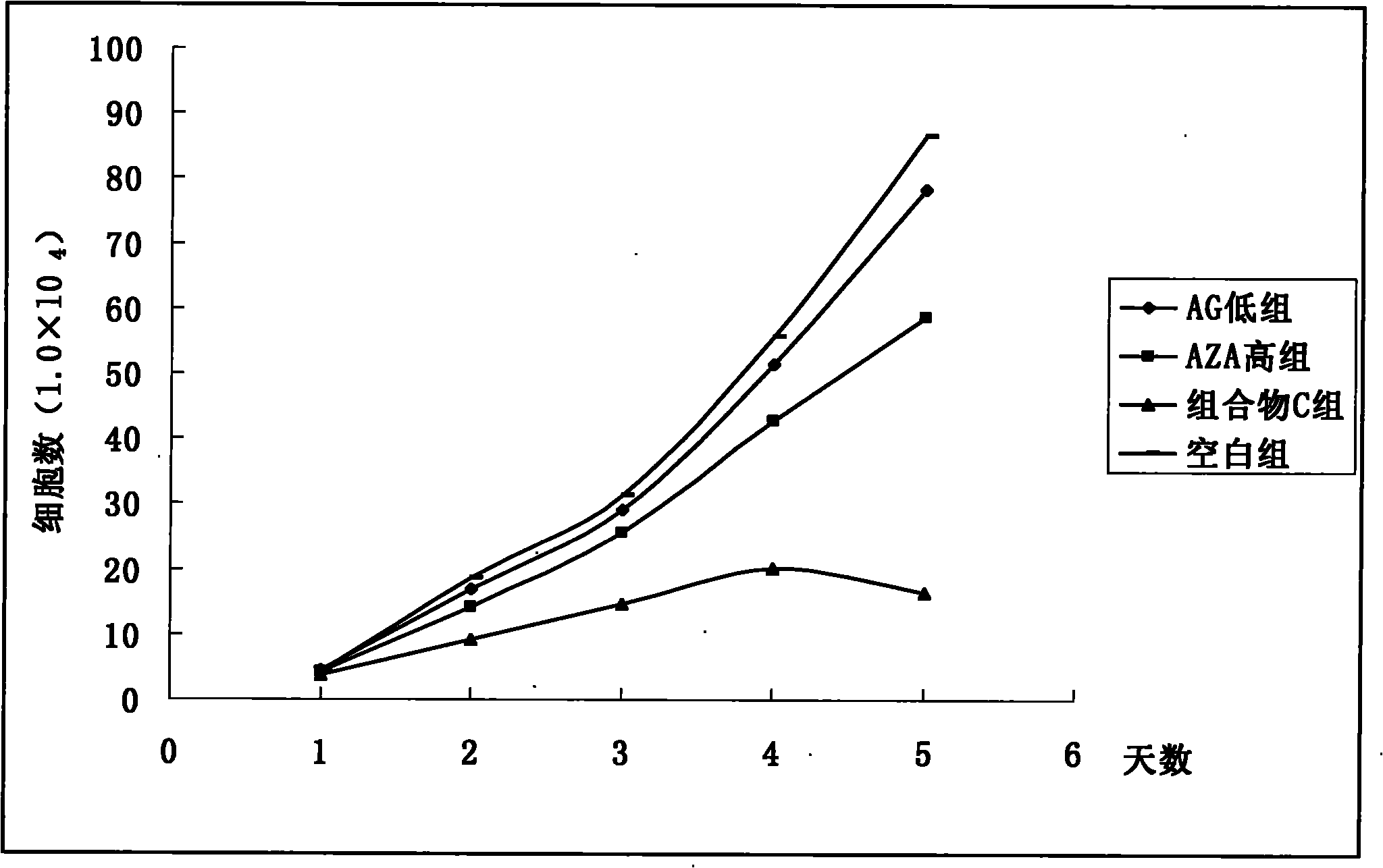
Pharmaceutical composition containing arctigenin and its medicinal use
A technology of arctigenin and composition, which is applied in the field of pharmaceutical composition containing arctigenin and its medical application, and can solve problems such as low curative effect, high recurrence rate, and functional inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1 injection
[0043] Arctigenin 20g
[0044] Decitabine 10g
[0045] Soybean oil for injection 380g
[0046] Glycerin 120g
[0047] Soy Lecithin 13g
[0048] Add water for injection to 2000ml
[0049] Preparation process: Disperse soybean lecithin and glycerin in 300ml water for injection, then mix arctigenin, decitabine and soybean oil for injection evenly to prepare an oil phase, then add the oil phase to the water phase, Stir while adding, after homogenization, sonicate for 1 hour, then dilute to 2000ml with water for injection, then transfer to a high-pressure homogenizer, emulsify for 30 minutes, fill, and sterilize to obtain.
Embodiment 2
[0050] Embodiment 2 injection
[0051] Arctigenin 30g
[0052] Decitabine 10g
[0053] Soybean oil for injection 360g
[0054] Glycerin 82g
[0055] Soy Lecithin 120g
[0056] Add water for injection to 2000ml
[0057] Preparation process: Disperse soybean lecithin and glycerin in 300ml water for injection, then mix arctigenin, decitabine and soybean oil for injection evenly to prepare an oil phase, then add the oil phase to the water phase, Stir while adding, after homogenization, sonicate for 1 hour, then dilute to 2000ml with water for injection, then transfer to a high-pressure homogenizer, emulsify for 30 minutes, fill, and sterilize to obtain.
Embodiment 3
[0058] Embodiment 3 injection
[0059] Arctigenin 10g
[0060] Decitabine 50g
[0061] Soybean oil for injection 120g
[0062] Glycerin 42g
[0063] Propylene glycol 16g
[0064] Soy Lecithin 5g
[0065] Add water for injection to 800ml
[0066] Preparation process: Disperse soybean lecithin, glycerin and propylene glycol in 120ml of water for injection, then mix arctigenin, decitabine and soybean oil for injection evenly to prepare an oil phase, and then add the oil phase to the water phase , stirring while adding, ultrasonication for 1 hour after homogenization, then dilute to 800ml with water for injection, then transfer to a high-pressure homogenizer, emulsify for 30 minutes, fill, and sterilize to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com