A tetrahydropyrazolopiperazine compound and its preparation method and application
A tetrahydropyrazole and compound technology, applied in the field of tetrahydropyrazolopiperazine small molecule compounds, can solve the problems of low yield, dose dependence, unsuitability for industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
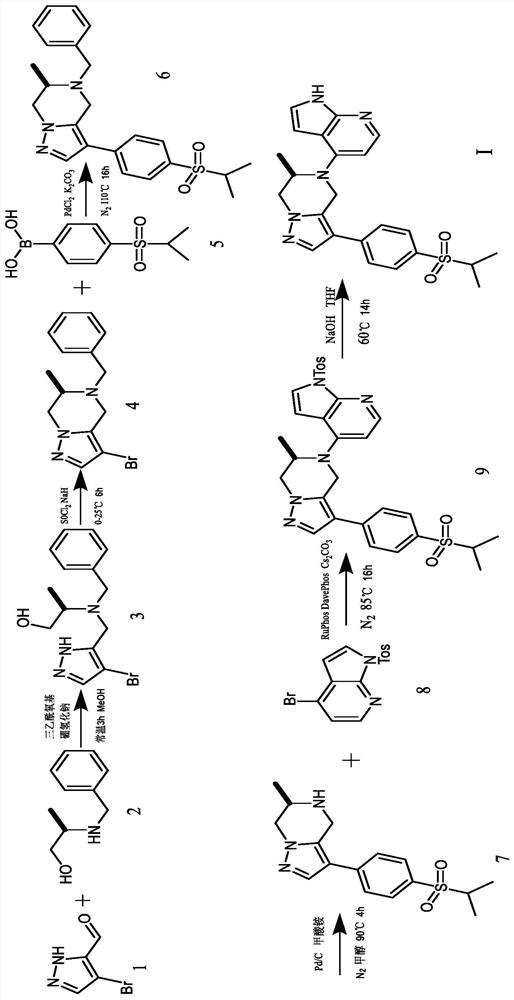
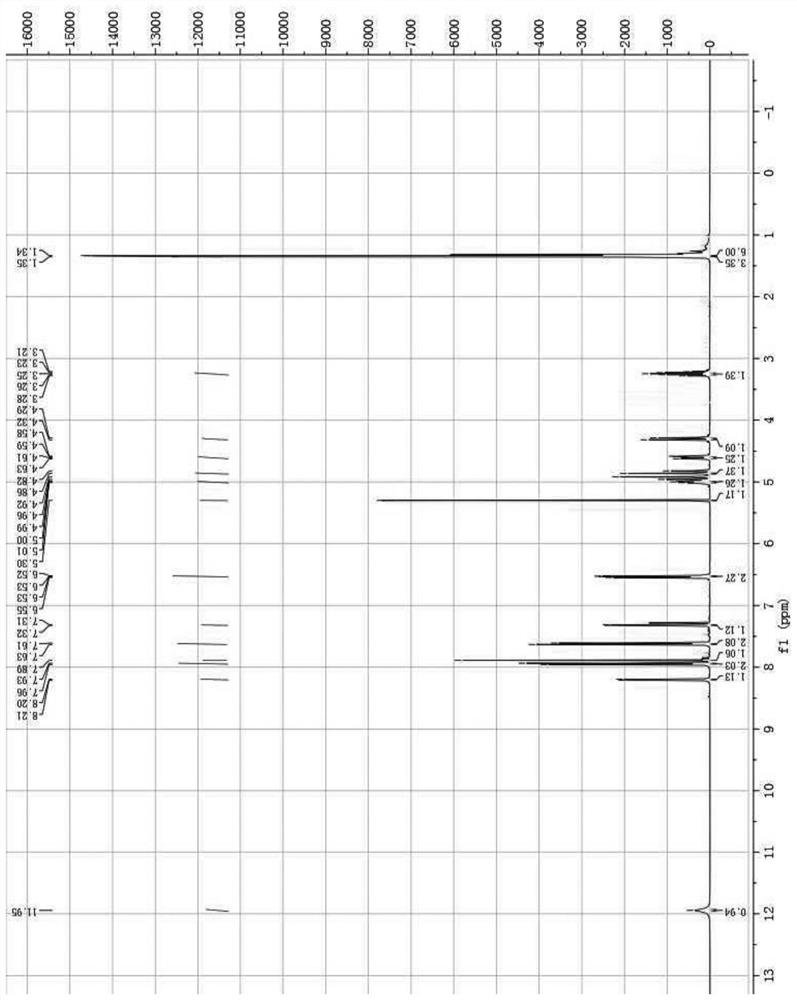
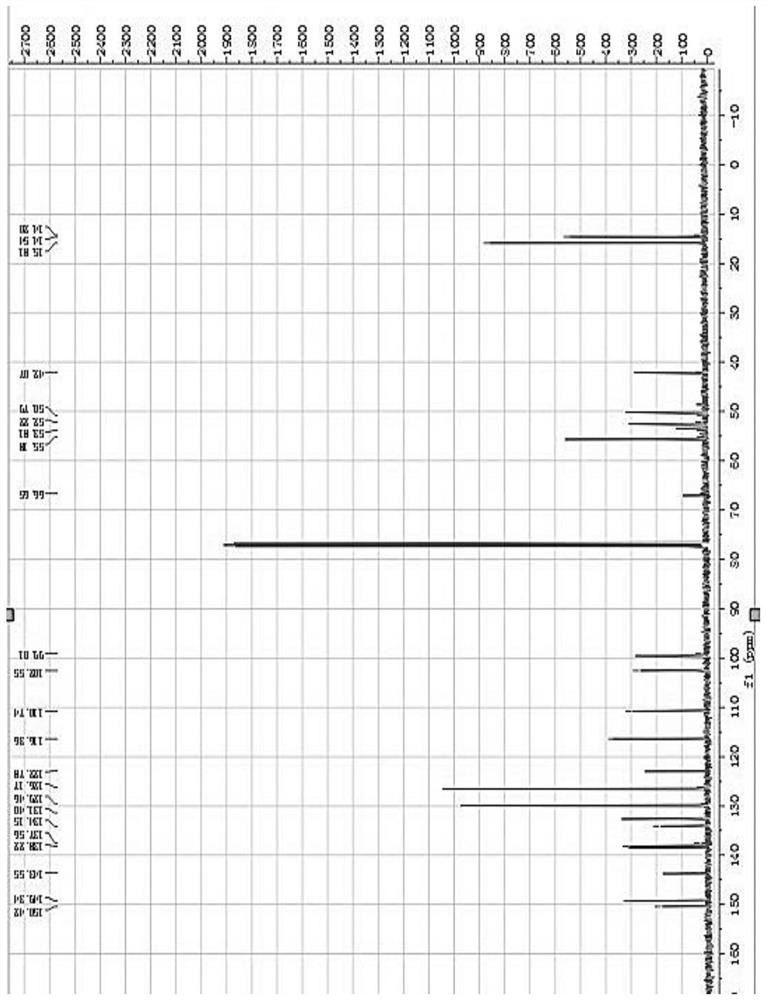
[0024] The present invention will be further described below through specific embodiments in conjunction with the accompanying drawings, and these embodiments are only used to illustrate the present invention, and are not intended to limit the protection scope of the present invention.
[0025] 3-Formyl-4-bromopyrazole (875mg, 5mmol) and (R)-2-(benzylamino)propan-1-ol (996mg, 6mmol) were added to methanol (20mL) solution, and then Sodium triacetoxyborohydride (3.18g, 15mmol) was added to the reaction mixture, reacted at room temperature for 3h, the reaction mixture was diluted with water, extracted 3 times with ethyl acetate, the organic phases were combined and washed with anhydrous Na 2 SO 4 After drying, the organic phase was filtered, concentrated under reduced pressure and purified by silica gel column to obtain the product (R)-2-(benzyl((4-bromo-1H-pyrazol-5-yl)methyl)amino)propan-1- Alcohol (1.13 g, 70% yield).
[0026] (R)-2-(Benzyl((4-bromo-1H-pyrazol-5-yl)methyl)am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com