High molecular adriamycin bonded medicament and preparation method thereof
A doxorubicin and polymer technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low drug loading, cumbersome preparation process, poor biocompatibility, etc. problem, achieve good biocompatibility, simple preparation process, and enhance the effect of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The present invention also provides a method for preparing a polymer doxorubicin-bonded drug, comprising the following steps:
[0054] a) polyethylene glycol monomethyl ether, carboxylated doxorubicin derivatives, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxysuccinimide is reacted in an organic solvent to obtain a reaction mixture, and the carboxylated doxorubicin derivative has formula (IV-a), formula (IV-b), formula (IV-c) or formula (IV-d) structure:
[0055]
[0056] In formula (III), n is the degree of polymerization, 10≤n≤120;
[0057]
[0058]
[0059] b) adding poly(L-lysine) or chitosan to the reaction mixture obtained in step a), and reacting to obtain a polymer doxorubicin-bonded drug.
[0060] The present invention uses poly(L-lysine) or chitosan, which has a wide source of raw materials and a simple preparation process, as a raw material, and the prepared polymer doxorubicin-bonded drug has a hydrophilic group and a hy...
Embodiment 5
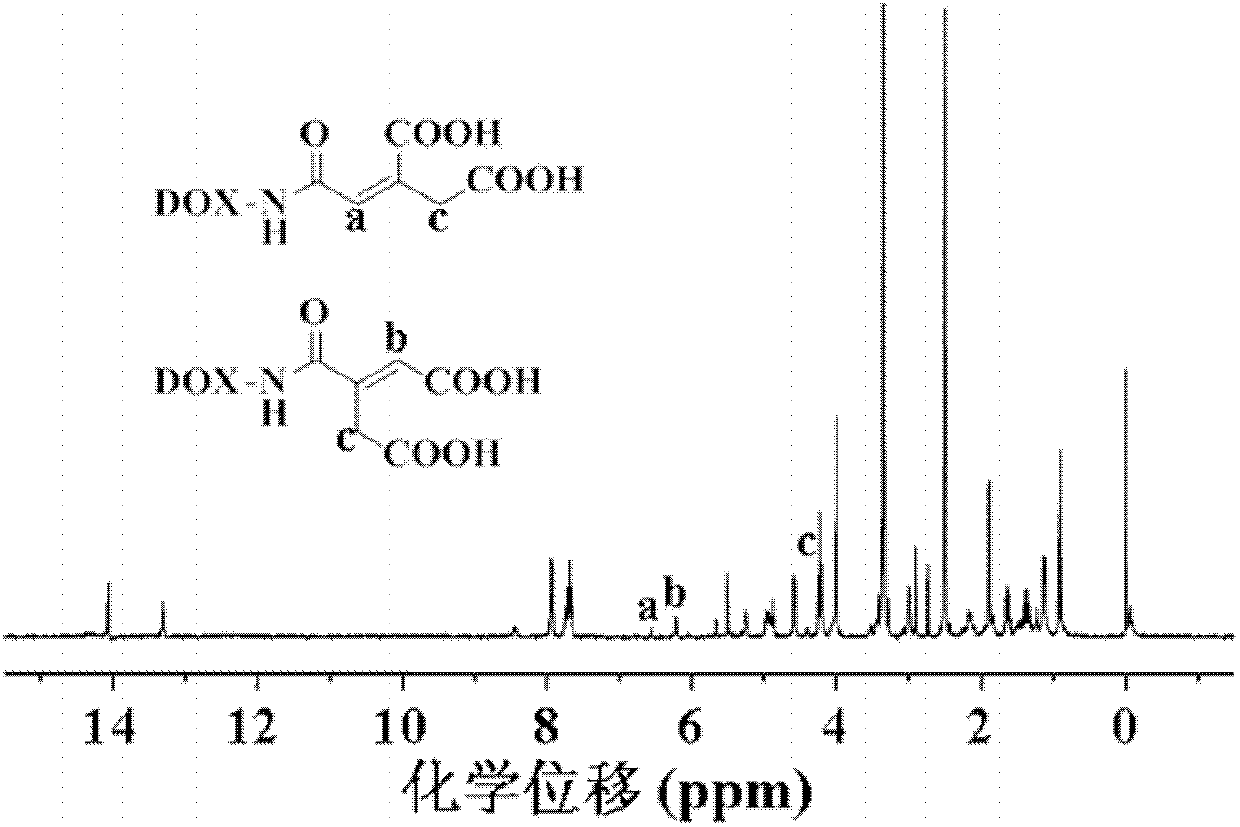
[0088] Weigh 580.0mg (0.001mol) doxorubicin hydrochloride, 156.1mg (0.001mol) cis-3-carboxyglutaconic anhydride and 101.2mg (0.001mol) triethylamine into a dry reaction flask, add Dissolve 5mL of anhydrous N,N-dimethylformamide and react at 25°C for 24h under stirring with a stirrer. Wash with aqueous sodium solution, dry, filter, and concentrate to obtain carboxylated doxorubicin derivatives.
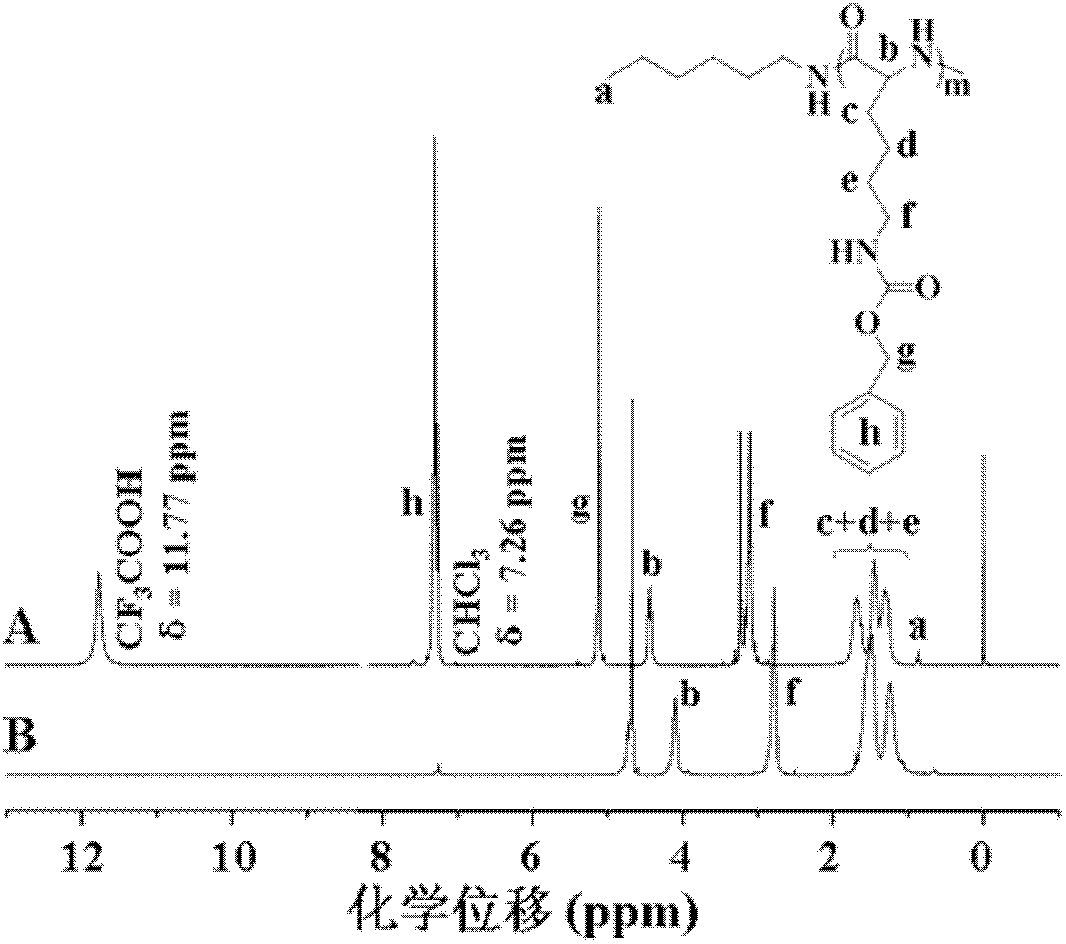
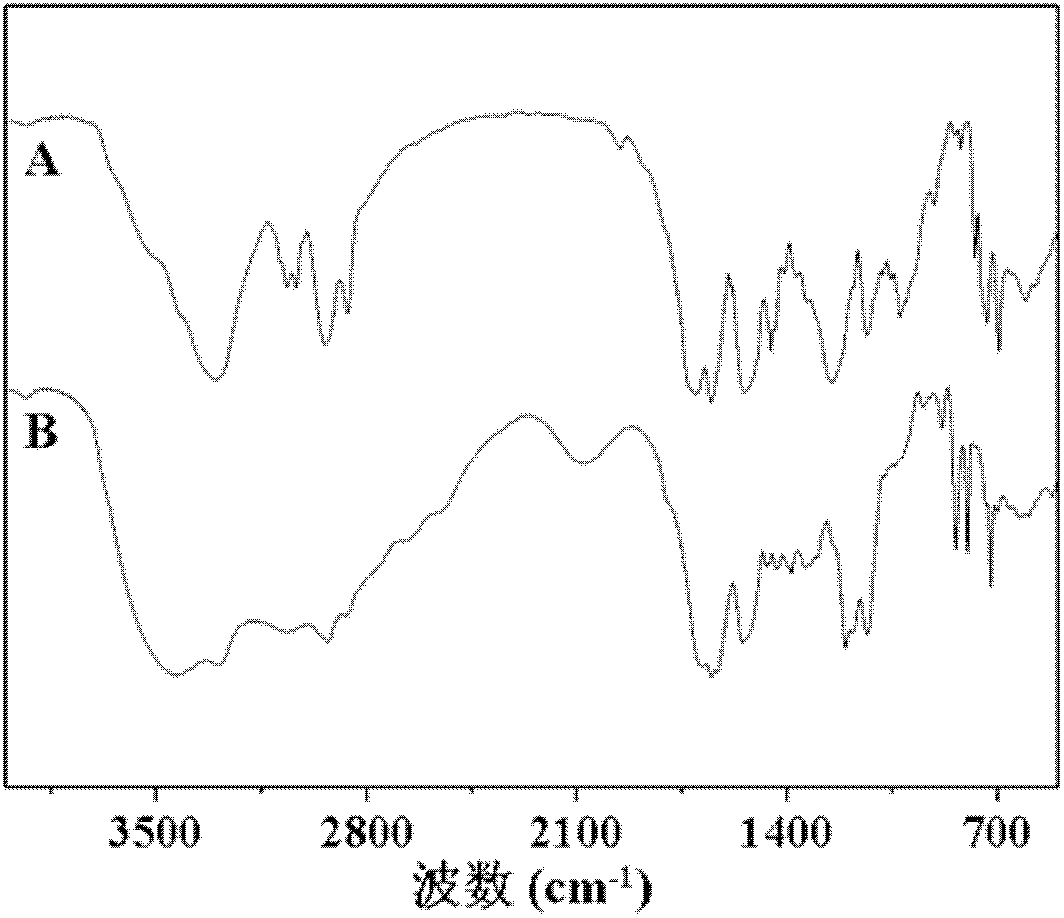
[0089] Carry out nuclear magnetic resonance analysis to the doxorubicin derivative of described carboxylation, the result sees image 3 , image 3 The H NMR spectrum of the carboxylated doxorubicin derivatives obtained in Example 5 of the present invention; infrared analysis is carried out on the carboxylated doxorubicin derivatives, and the results can be found in Figure 4 , Figure 4 For the infrared analysis spectrogram of the carboxylated doxorubicin derivative obtained in Example 5 of the present invention, by image 3 and Figure 4 It can be seen that the carboxylated doxor...
Embodiment 6
[0090] The synthesis of the doxorubicin derivative of embodiment 6 carboxylation
[0091] Weigh 580.0mg (0.001mol) doxorubicin hydrochloride, 152.2mg (0.001mol) 1,2-dicarboxycyclohexene anhydride and 101.2mg (0.001mol) triethylamine into a dry reaction flask, add Dissolve 5mL of anhydrous N,N-dimethylformamide and react at 25°C for 24h under stirring with a stirrer. After washing with aqueous sodium solution, drying, filtering and concentrating, carboxylated doxorubicin derivatives are obtained.
[0092] The nuclear magnetic resonance analysis and infrared analysis of the carboxylated doxorubicin derivatives show that the carboxylated doxorubicin derivatives prepared in the examples of the present invention have the structure of formula (IV-a).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap