Antibodies against human CCN1 and uses thereof
An antibody and application technology, applied in the field of antibodies against human CCN1 and its application, can solve the problems of mucin, loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] immunity
[0069] a) Immunization of mice with human mutant CCN1
[0070] 50 μg of recombinant human CCN1 containing a single amino acid substitution E173D or F185L was injected intraperitoneally with complete Freund's adjuvant on day 0, day 28 and day 56 (both with incomplete Freund's adjuvant) and Balb / c and NMRI mice were immunized with 50 μg of recombinant protein on day 84 together with incomplete Freund's adjuvant. Blood was collected on days 91 and 108 and serum was prepared for titer determination by ELISA (see below). Animals with the highest titers were selected for boosting on day 112 by intravenous injection of 50 μg of recombinant human CCN1.
[0071] b) Immunization of NZW rabbits with human mutant CCN1
[0072] 100 μg of recombinant human CCN1 containing a single amino acid substitution E173D or F185L was used on day 0 with complete Freund's adjuvant and with 100 μg protein on day 21, day 43, day 65 and day 85 with incomplete Freund's adjuvant immuniz...
Embodiment 2
[0082] Recombinant expression of membrane-bound CCN1 and CCN1 domains
[0083] Adherently grown cells were transfected with a recombinant vector encoding CCN1 or each CCN1 domain fused at the C-terminus to the transmembrane domain of the PDGF receptor (human platelet-derived growth factor receptor beta-type, UniProt accession number P09619, amino acids 513 to 561). Mouse NIH 3T3 cells (CRL-1658 TM ) or suspension-adapted human HEK293 (CRL-1573 TM )cell.
[0084] Cell binding assays, measured by FACS, were performed using suspension-adapted HEK293 cells. Adherently grown NIH 3T3 cells were used for cell binding assays analyzed in fluorescence microscopy.
Embodiment 3
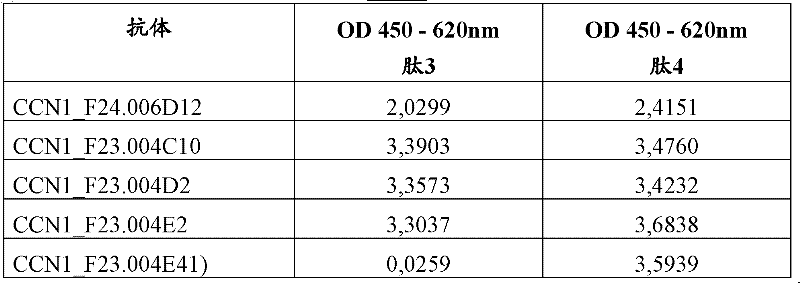
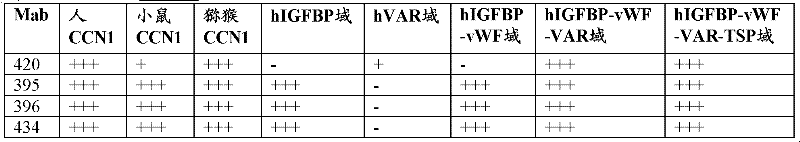
[0086] Antibody binding to human CCN1 and fragments determined by ELISA
[0087] Human and mouse CCN1 ELISA
[0088] Antibody binding to human and mouse CCN1 was determined by ELISA. Recombinant human CCN1 or mouse recombinant CCN1 containing single amino acid substitutions E173D or F185L were immobilized on 384-well Nunc Maxisorp plates at 2.5 μg / ml in PBS, 25 μl / well by incubation overnight at 2-8°C. Plates were blocked with PBS / 1% BSA for 1 hour at room temperature, followed by two washing steps (0.1% Tween-20 in PBS), and incubated at room temperature with different concentrations of anti-CCN1 antibody or hybridomas in blocking buffer. The supernatants were incubated together for 1 hour. After washing four more times, anti-mouse-HRP (Amersham #NA9310), anti-rabbit-HRP (Jackson Immunoresearch #711-036-152) or anti-human IgG-HRP (Jackson Immunoresearch #109-036-097) was used to detect antibodies for 1 hour at room temperature. Signals were visualized by adding 25 μl TMB ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap