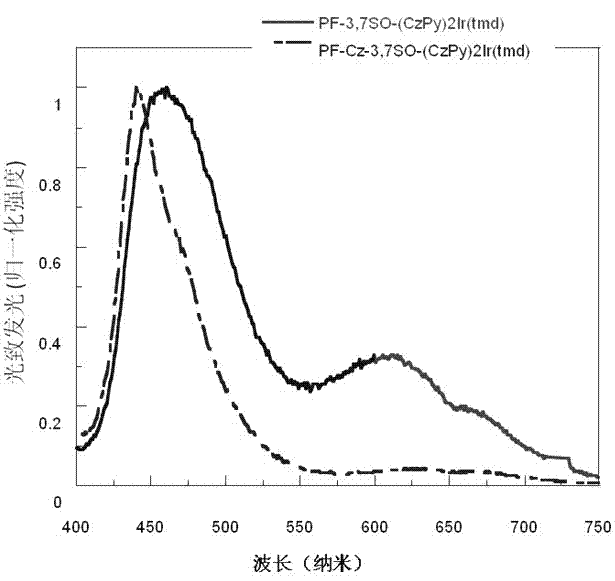
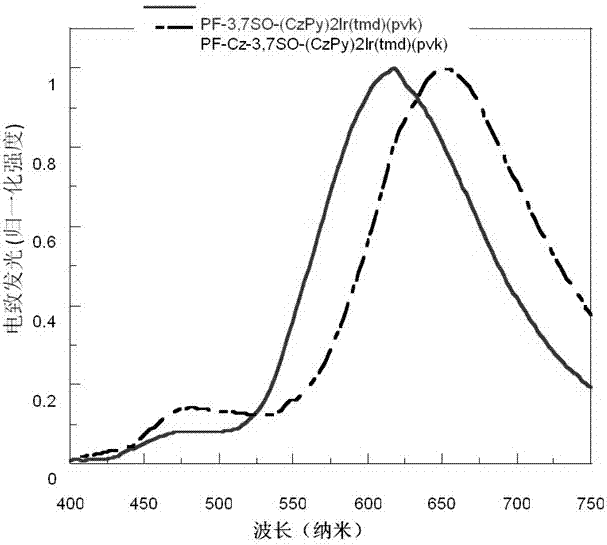
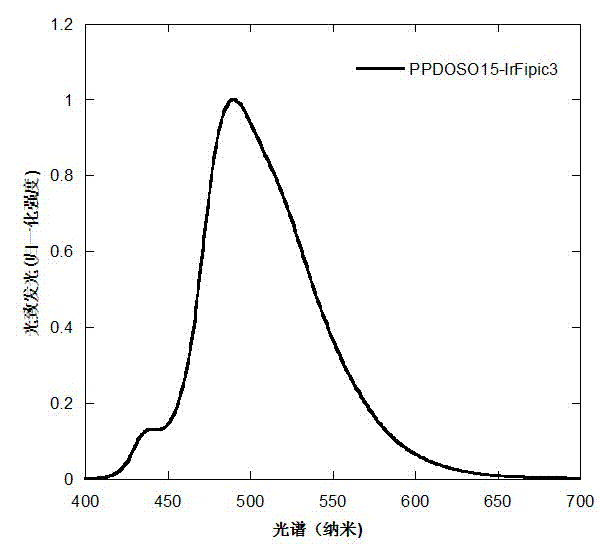
Electro-phosphorescence polymer containing (alkyl-substituted-)S,S- dioxo-dibenzothiophene unit and application thereof
A dibenzothiophene and polymer technology, applied in the application field of electrophosphorescent polymers and preparation of light-emitting devices, can solve problems such as reduction, achieve broad application prospects, good stability, and improve the balance of injection and transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: 3, the preparation of 7-dibromo-S, S-dioxy-dibenzothiophene
[0057] 1)
[0058]
[0059] In a 150ml round bottom flask, add dibenzothiophene (9.2g, 50mmol), acetic acid (100ml), 35% concentration of hydrogen peroxide (30mL), and then react under reflux for 6 hours. After the reaction, the reactant was poured into water, filtered, and washed several times with methanol. After drying, it was recrystallized with ethanol to obtain colorless needle crystals, 9.7 g, yield: 90%.
[0060] 2)
[0061]
[0062] In a 100ml single-necked bottle, add S, S-dioxy-dibenzothiophene (5.4g, 25mmol), concentrated sulfuric acid (50ml), slowly add bromosuccinimide (11.1ml, 62.5mmol), room temperature Under reaction for 24 hours. After the reaction was over, the reactant was poured into 500ml of crushed ice, and the ice was melted with NaCO 3 The solution was adjusted to be neutral, and the insoluble matter was filtered out, washed with water and dried, and then re...
Embodiment 2
[0063] Example 2: 2,8-dioctyl-3,7-diboronate-S, the preparation of S-dioxo-dibenzothiophene 1)
[0064]
[0065] In a 100ml three-necked flask, add dibenzothiophene (5.5g, 30mmol) and iron powder (0.084g, 1.5mmol) and dissolve in 30mL of chloroform, avoid light, add 3.1mL of liquid bromine at 0-5°C, Reaction at room temperature for 20h. Add saturated NaHSO 3 The excess bromine was removed by aqueous solution, and a pale yellow solid was obtained by filtration, washed twice with methanol, and recrystallized from chloroform to obtain a white solid. Yield: 80%.
[0066] 2)
[0067]
[0068] In a 100 ml three-necked flask, add 2,8-dibromo-dibenzothiophene (6.48g, 20mmol), Ni(dppp)Cl 2 (1.08g, 0.2mmol), anhydrous tetrahydrofuran (200mL), protected from light, 10 ℃ dropwise added C 8 h 17 MgBr in diethyl ether (45 mmol). After the dropwise addition was completed, remove the ice bath, react at room temperature for 2 h, and use saturated NH 4 Cl to remove excess Grignar...
Embodiment 4
[0079] Example 4: Preparation of 2,7-diboronate-9,9-di-n-octylfluorene
[0080] 1)
[0081]
[0082] In a 250 ml three-necked flask, add fluorene (16.6 g, 100 mmol), iron powder (2.8 g, 5 mmol), and 100 ml of chloroform. After cooling in an ice-water bath, 35 ml of bromine (35.2 g, 220 mmol) / chloroform mixed solution was added dropwise. The temperature in the bottle does not exceed 5°C during the dropwise addition. After the reaction was completed, it was filtered and recrystallized from chloroform to obtain 26.9 g of white crystals with a yield of 83%.
[0083] 2)
[0084]
[0085] Add 2,7-dibromofluorene (9.7g, 30mmol), benzyltriethylammonium chloride (0.07g, 0.3mmol), dimethyl sulfoxide 90mL, 45mL sodium hydroxide aqueous solution (50mL) into a 100mL three-necked flask %). Stir vigorously at room temperature to form a suspension. 1-Bromo-n-octane (12.5 g, 65 mmol) was slowly added dropwise, and after stirring was continued for 3 hours, it was extracted with di...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap