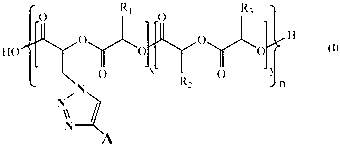
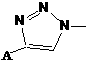
Biological functionalization degradable polyester and preparation method thereof
A technology for biofunctionalization and degradation of polyester, applied in organic chemistry, bulk chemical production, etc., can solve problems such as unsatisfactory biocompatibility and difficult high-density biological modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
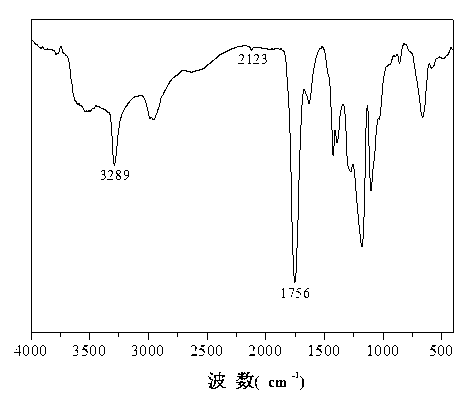
[0052] like figure 1 As shown, 1756cm -1 The characteristic infrared absorption peak of ester carbonyl is at 3289cm -1 and 2123 cm -1 The characteristic infrared absorption peak of the alkynyl group is at the place, and the results show that the steps (1) and (2) synthesized the alkynylated lactide derivative monomer.
[0053] Step (3): Weigh 12 g of the alkynylated glycolide derivative crystal obtained in step (2), and add 0.5 w The stannous octoate of t% is polymerized under the assistance of microwave, wherein: setting reaction temperature is 100 ℃, and microwave power is 30W, and reaction time is 20min; Solid crude product is dissolved with chloroform, dehydrated alcohol precipitation, then in 45 °C for 24 hours in vacuum to obtain a side chain alkynylated biodegradable polyester (yield: 93.5%, number average molecular weight: 1.08*10 4 ).
[0054] Step (4): Weigh side-chain alkynylated biodegradable polyester (10.8g, 1.0mmol) and azide cholesterol (6.2g, 15.0mmol) i...
Embodiment 2
[0059] Step (3): Weigh 6 g of alkynylated glycolide derivative crystals obtained in step (2) and 9 g of lactide, and add 0.5 w t% of 4-dimethylaminopyridine, polymerized under microwave assistance, wherein: set the reaction temperature to 40°C, the microwave power to 50W, and the reaction time to 35min; the solid crude product was dissolved in chloroform and precipitated in absolute ethanol , and then vacuum-dried at 45°C for 24 hours to obtain a side chain alkynylated biodegradable copolyester (yield: 94.3%, number average molecular weight: 3.17*10 4 ).
[0060] like figure 2 As shown, 1757cm -1 The characteristic infrared absorption peak of ester carbonyl is at 3290cm -1 and 2120 cm -1 The place is the characteristic infrared absorption peak of the alkynyl group; image 3 The chemical shifts at 1.4 and 1.6 are the proton resonance peaks of the methyl group on the lactyl unit in the terminal and the chain segment respectively, 4.4 and 5.2 are the proton resonance peaks ...
Embodiment 3
[0066] Step (3): Weigh 3 g of azide glycolide derivative crystals obtained in step (2) and 9 g of caprolactone, and add 1.0 w t% of stannous octoate was polymerized by the bulk sealing tube method, wherein: the reaction temperature was set at 100°C, and the reaction time was 24h; the solid crude product was dissolved in chloroform, precipitated with anhydrous methanol, and then vacuum-dried at 40°C for 36h , to obtain a side chain azide biodegradable copolyester (yield: 93.6%, number average molecular weight: 2.98*10 4 ).
[0067] Step (4): Weigh side chain azide biodegradable copolyester (2.98g, 0.1 mmol) and alkynylated chitohexaose (1.5g, 1.5 mmol) into a 50ml three-neck flask, add 30ml of di Methyl sulfoxide was used to completely dissolve the raw materials, and copper bromide (CuBr, 21.6 mg, 0.15 mmol) and pentamethyldivinyltriamine (PMEDTA, 30 μL, 0.15 mmol) were added under nitrogen protection, and reacted at 25 °C for 36 h . After the reaction, the polymerization sy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com