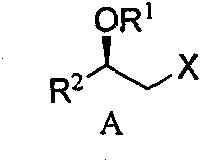
Intermediate for preparing rosuvastatin and preparation method and application thereof
A technology for the use of rosuvastatin, which is applied in the field of heterocyclic chemistry containing 1, can solve the problems of severe equipment corrosion, difficult separation and purification, and difficulty in industrialization, and achieves improved total yield, high reaction yield, and short reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of formula 2-1 compound
[0065]
[0066] In a 500ml three-necked flask equipped with a thermometer, nitrogen atmosphere and a constant pressure dropping funnel, add iodine (66g, 260mmol), 100ml of dichloromethane, stir at room temperature, dropwise add hexamethyldisilane (40g, 280mmol), dropwise After completion, keep stirring at 15-30°C for 30 minutes, raise the temperature to 45-70°C and react for 2-4 hours until the system becomes colorless and clear, cool to -20-0°C, add R-epichlorohydrin (36g, 400 mmol) of dichloromethane solution, stirred at this temperature for 3 to 10 hours after the dropwise addition, and evaporated to dryness to obtain 110 g of the compound shown in Formula 2-1 as a slightly yellowish liquid with a yield of 98%.
[0067] 1 HNMR (400MHz, CDCl 3 ): δ=0.190 (S, 9H), δ=3.26-3.298 (dd, 1H, J=4.8, 10.4Hz), δ=3.322-3.362 (dd, 1H, J=4.8, 10.4Hz), δ=3.518 -3.601 (m, 2H), δ=3.753-3.806 (m, 1H).
[0068] MS(EI+): 292...
Embodiment 2
[0069] Embodiment 2: the preparation of formula 2-2 compound
[0070]
[0071] In a 500ml three-necked flask equipped with a thermometer, nitrogen atmosphere and a constant pressure dropping funnel, add iodine (66g, 260mmol), 100ml of dichloromethane, stir at room temperature, dropwise add hexamethyldisilane (40g, 280mmol), dropwise After completion, keep stirring at 15-30°C for 30 minutes, raise the temperature to 45-70°C and react for 2-4 hours until the system becomes colorless and clear, cool to -20-0°C, add R-epibromopropane (54g, 394mmol) of dichloromethane solution, stirred at this temperature for 2.5 to 12 hours after the dropwise addition, and evaporated to dryness to obtain 127g of the compound shown in formula 2-2 as a light yellow liquid with a yield of 96%.
[0072] MS(EI+): 338[M+], 336[M+].
Embodiment 3
[0073] Embodiment 3: the preparation of formula 3-1 compound
[0074]
[0075] Equip a 500ml single-necked bottle with a stirrer, add the compound of formula 2-1 (110g, 400mmol), triphenylphosphine (20g, 75mmol), heat up to 70-90°C and stir for 10-15 hours, cool, add 50-200ml of toluene , filtered, and the filtrate can be reused after precipitation. The filter cake was washed with 20-50 ml of toluene and dried to obtain 28 g of the compound shown in Formula 3-1 as a yellowish solid with a yield of 90%.
[0076] 1 HNMR (400MHz, DMSO): δ=0.289(S, 9H), δ=3.766-3.841(m, 2H), δ=3.847-3.941(m, 1H), δ=4.172-4.220(m, 1H), δ =4.260-4.355 (m, 1H).
[0077] MS(EI+): 554[M+].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com