Hydroxyl phosphate amino acid ester derivatives of Brefeldin A, preparation method thereof, and application thereof
A technology of hydroxyphosphate amino acid ester and hydroxyphosphate amino acid, which can be applied in the field of fungal metabolites and can solve problems such as low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
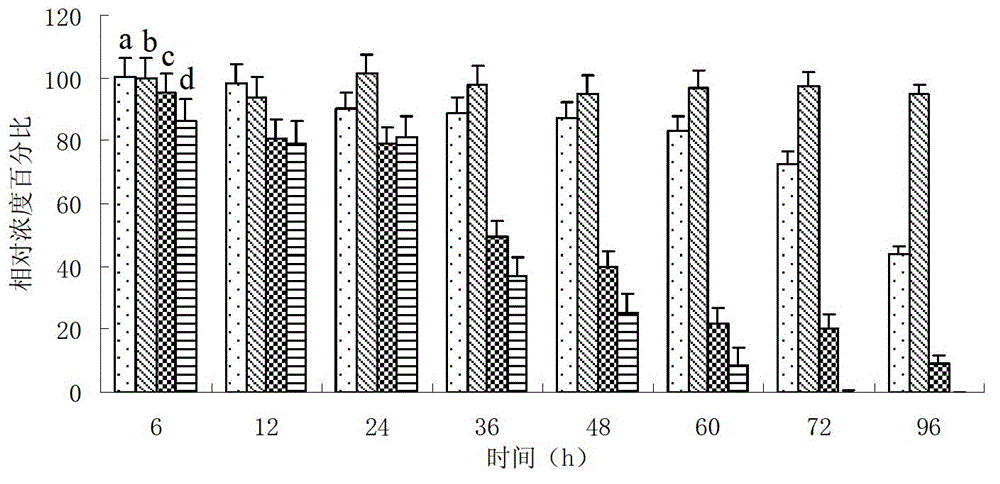
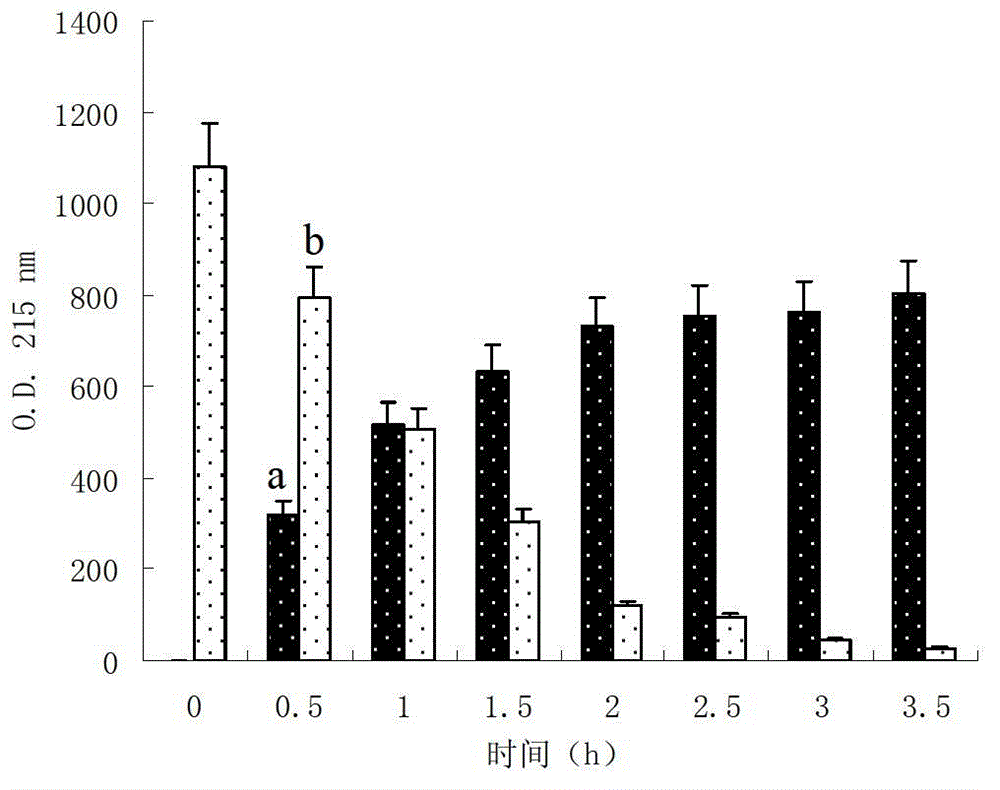
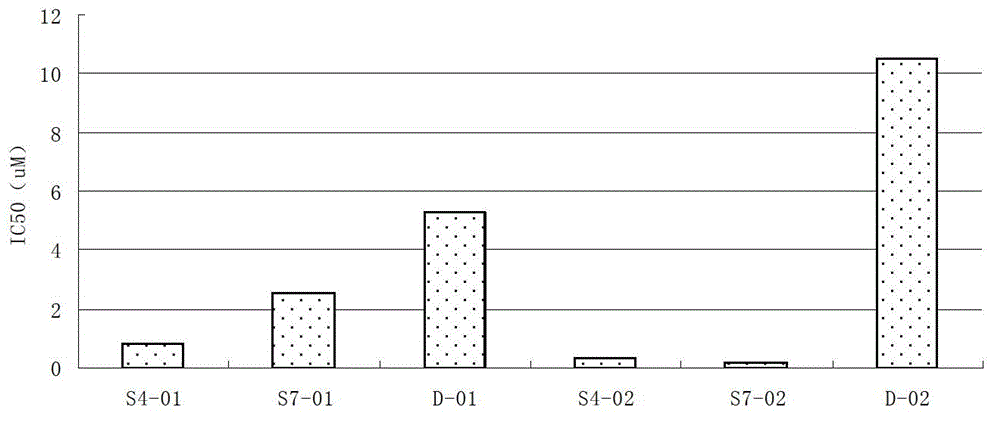
Image
Examples
Embodiment 1
[0060] In a 25mL round bottom flask, add 0.5mmol of (+)-Brefeldin A, 2mmol of phosphorylated alanine, 0.05mmol of 4-dimethylaminopyridine, add 10mL of anhydrous dichloromethane under argon protection, Add 2 mmol of dicyclohexylcarbodiimide dropwise to the reaction system under ice-water bath 2 Cl 2 After the solution was added dropwise, it was stirred overnight at room temperature, and after the completion of the reaction was monitored by thin-layer chromatography, the insoluble matter (DCU) was filtered off, and the filtrate was concentrated under reduced pressure. Using V (petroleum ether): V (ethyl acetate) = 1: 3, the pure product (D-02) was obtained with a yield of 90%. (spectral data, 31 P-NMR (CDCl 3 ):δ=5.08,4.93ppm; 1 H-NMR (400MHz, CDCl 3 ):δ7.21(dd,J=15.9,3.5Hz,1H),5.66-5.78(m,2H),5.13-5.31(m,3H),4.82-4.93(m,1H),4.53-4.66(m ,4H),3.94-4.03(m,1H),3.82-3.92(m,1H),3.20(q,J=8.7Hz,2H),2.41-2.53(m,1H),2.30-2.40(m,1H ),1.99-2.21(m,4H),1.80-1.86(m,1H),1.68-1.78(m,2H),...
Embodiment 2
[0062] Dissolve 280 mg (1 mmol) of (+)-Brefeldin A and 82 mg (1.2 mmol) of imidazole in 10 mL of dry DMF, and cool in an ice-water bath. 165 mg (1.1 mmol) of TBSCl was dissolved in 5 mL of dry DMF, and the solution was slowly added dropwise to the above-mentioned system with a syringe, stirred at room temperature for 24 h, and after the TLC plate monitored that the reaction was complete, 20 mL of an organic solvent ( II) After dilution, wash with 3 x 10 mL of water. The organic phase was concentrated under reduced pressure. Use V (petroleum ether): V (ethyl acetate) = 8: 1 to pass through the column to obtain the pure product (+)-BrefeldinA protected by TBS at position 7 (code: BFA-7TBS), with a yield of 86%. Add 0.5mmol of BFA-7TBS, 1mmol of phosphorylated alanine, and 0.05mmol of 4-dimethylaminopyridine into a 25mL round bottom flask, add 10mL of anhydrous dichloromethane under the protection of argon, and place in an ice-water bath Add 1 mmol of dicyclohexylcarbodiimide i...
Embodiment 3
[0064] Add 0.5mmol of (+)-Brefeldin A protected by TBS at position 4, 1mmol of DIPP-Ala, 0.05mmol of 4-dimethylaminopyridine into a 25mL round-bottomed flask, add 10mL of anhydrous di Chloromethane, add 1mmol of CH dicyclohexylcarbodiimide dropwise to the reaction system under ice-water bath 2 Cl 2 After the solution was added dropwise, it was stirred overnight at room temperature, and after the completion of the reaction was monitored by thin-layer chromatography, the insoluble matter (DCU) was filtered off, and the filtrate was concentrated under reduced pressure. Using petroleum ether: ethyl acetate at a ratio of 1:3 to pass through the column, the yield of pure product was 92%. In a 10 mL round bottom flask, add 0.3 mmol of (+)-Brefeldin A-7-hydroxy phosphate amino acid ester protected by TBS at position 4, add 5 mL of CH 3 OH, after the product dissolves, add 0.02mg of I to the reaction system 2 , react at 60°C for 16h, after the reaction is complete, add 1mol / L Na dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com