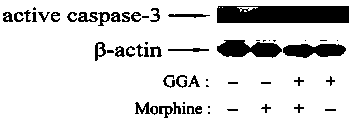Use of geranylgeranylacetone for preventing and treating renal injuries caused by morphine
A technology for teprenone and kidney damage, applied in the field of medicine, to achieve high safety, reduce economic burden, and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Teprenone inhibits morphine-induced caspase-9 activation in the kidney
[0024] The mice were divided into 4 groups (6 in each group): control group (saline), morphine group (Mor), GGA and morphine group (GGA+Mor), GGA group. From day 1 to day 7, the Saline group and Mor group were given normal saline in advance by gavage, GGA+Mor group and GGA group were given GGA (800 mg / kg) by gavage in advance, and the Saline group and GGA group were given intraperitoneal injection 2 hours later. Saline, Mor group and GGA+Mor group were intraperitoneally injected with morphine (the daily doses were 10, 20, 40, 60, 80, 100 and 100 mg / kg). Two hours after the last morphine injection, the kidney tissues of the mice were separated, the protein was extracted, and the expression of pro-caspase-9 in the kidney cells was detected by Western blotting. figure 1 The results showed that pre-perfusion of GGA can inhibit the decrease of pro-caspase-9 caused by morphine, which indicates t...
Embodiment 2
[0025] Example 2: Teprenone inhibits morphine-induced caspase-3 activation in the kidney
[0026] The mice were divided into 4 groups (6 in each group): control group (saline), morphine group (Mor), GGA and morphine group (GGA+Mor), and GGA group. From day 1 to day 7, the Saline group and Mor group were given normal saline in advance by gavage, GGA+Mor group and GGA group were given GGA (800 mg / kg) by gavage in advance, and the Saline group and GGA group were given intraperitoneal injection 2 hours later. Saline, Mor group and GGA+Mor group were injected intraperitoneally with morphine (the daily doses were 10, 20, 40, 60, 80, 100 and 100 mg / kg). Two hours after the last morphine injection, the kidney tissues of the mice were separated, the protein was extracted, and the expression of caspase-3 in kidney cells was detected by Western blotting. figure 2 The results show that pre-perfusion of GGA can inhibit the increase of active caspase-3 caused by morphine, which indicates tha...
Embodiment 3
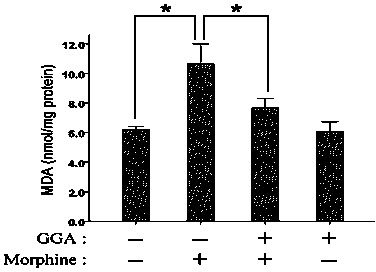
[0027] Example 3: Teprenone inhibits the increase in malondialdehyde (MDA) caused by morphine in the kidney
[0028] The mice were divided into 4 groups (6 in each group): control group (saline), morphine group (Mor), GGA and morphine group (GGA+Mor), and GGA group. From day 1 to day 7, the Saline group and Mor group were given normal saline in advance by gavage, GGA+Mor group and GGA group were given GGA (800 mg / kg) by gavage in advance, 2 hours later, the Saline group and GGA group were injected intraperitoneally Physiological saline, Mor group and GGA+Mor group were intraperitoneally injected with morphine (daily injection dose: 10, 20, 40, 60, 80, 100 and 100 mg / kg). Two hours after the last morphine injection, the mouse kidney tissue was separated, prepared into a 10% homogenate with phosphate buffer in an ice bath, and centrifuged in a high-speed refrigerated centrifuge for 10 minutes (4 ℃, 4000 rpm), Take the supernatant and use the malondialdehyde (MDA) ELISA kit to de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com