Detection method for meclozine hydrochloride tablet-related substances
A technology of meclizine hydrochloride tablets and meclizine hydrochloride, which can be used in measurement devices, instruments, scientific instruments, etc., can solve the problems of chromatographic column damage, large mobile phase, large concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
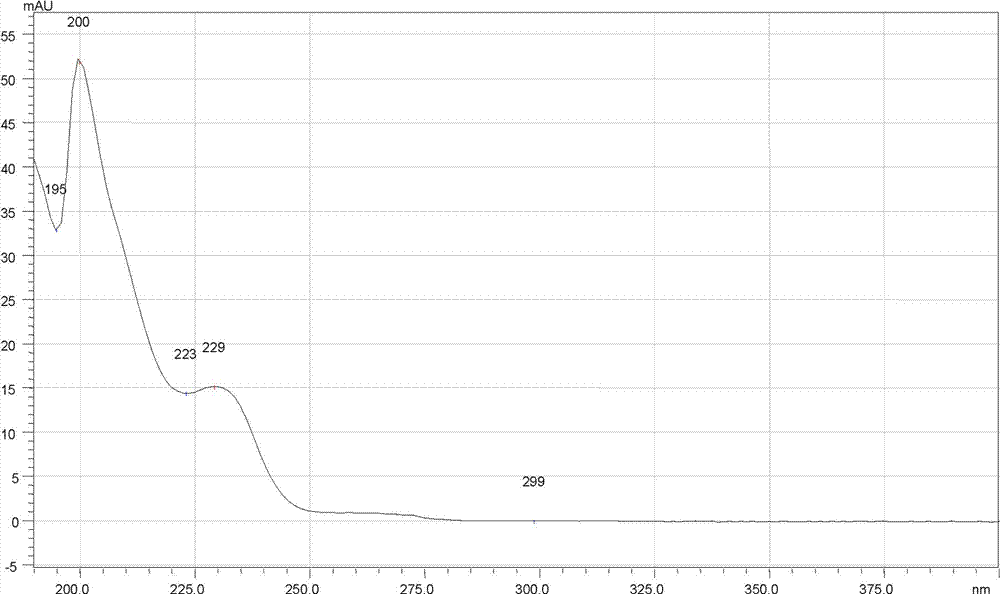
[0097] The chromatographic conditions are: the chromatographic column is Kromasil 100-5 phenyl chromatographic column, the specification is 250 mm×4.6 mm, 5 μm; the mobile phase: A is methanol, B is diammonium hydrogen phosphate solution with pH 7.0; the gradient elution program is 0→5.0min, A is 80%; 5.0→35.0min, A changes from 80% to 95%; 35.0→35.01min, A changes from 95% to 80%; 35.01→45.0min, A is 80%; flow rate 1.0 ml / min; detection wavelength: 225 nm; column temperature: 30°C; theoretical plate number based on meclizine hydrochloride should not be less than 2000;
[0098] Determination method: 10 tablets of this product, grind finely, take the powder equivalent to 50mg of meclizine hydrochloride, accurately weigh it, put it in a 50ml volumetric flask, use the mixed solution of mobile phase A and B as the diluent, wherein A is 80% , dissolve with a diluent and dilute to the mark, shake well, filter, and take the subsequent filtrate as the test solution; accurately weigh t...
Embodiment 2
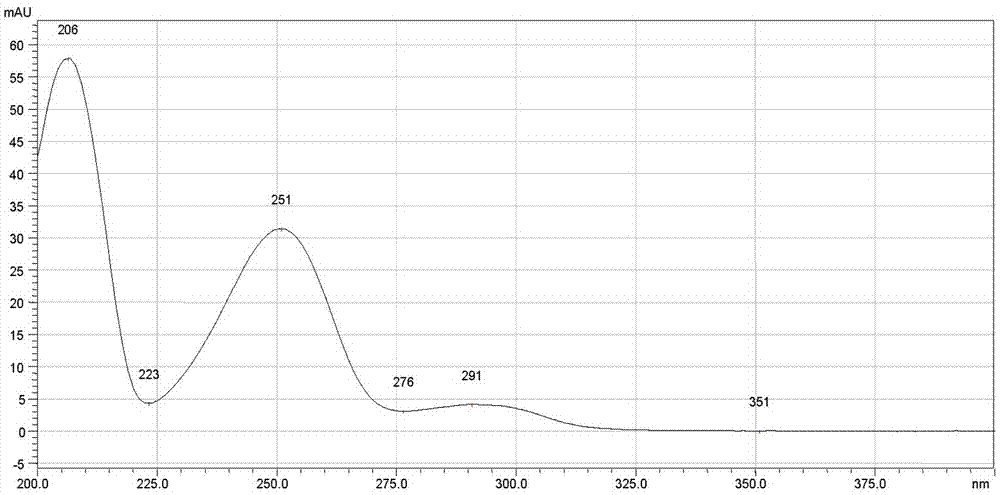
[0102] The chromatographic conditions are: the chromatographic column is a Crest Phenyl phenyl column, the specification is 250mm×4.6 mm, 5 μm; the mobile phase: A is methanol, B is diammonium hydrogen phosphate solution with pH 7.0; the gradient elution program is 0→5.0 min, A is 80%; 5.0→35.0min, A changes from 80% to 95%; 35.0→35.01min, A changes from 95% to 80%; 35.01→45.0min, A is 80%; flow rate 1.0ml / min ; Detection wavelength: 225 nm; Column temperature is 30°C; The number of theoretical plates should not be less than 2000 based on meclizine hydrochloride;
[0103] Determination method: 10 tablets of this product, grind finely, take the powder equivalent to 50mg of meclizine hydrochloride, accurately weigh it, put it in a 50ml volumetric flask, use the mixed solution of mobile phase A and B as the diluent, wherein A is 60% , dissolve with a diluent and dilute to the mark, shake well, filter, and take the subsequent filtrate as the test solution; accurately weigh the mec...
Embodiment 3
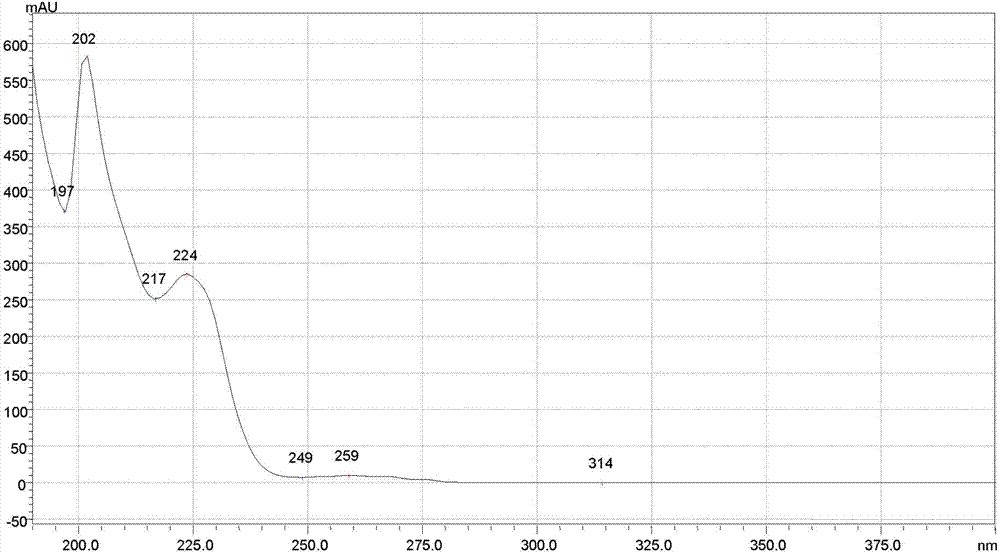
[0107] The chromatographic conditions are: the chromatographic column is a YMC-Pack phenyl chromatographic column, the specification is 250 mm×4.6 mm, 5 μm; the mobile phase: A is methanol, B is diammonium hydrogen phosphate solution with pH 7.0; the gradient elution program is 0→5.0min, A is 80%; 5.0→35.0min, A changes from 80% to 95%; 35.0→35.01min, A changes from 95% to 80%; 35.01→45.0min, A is 80%; flow rate 1.0 ml / min; detection wavelength: 225 nm; column temperature: 30°C; theoretical plate number based on meclizine hydrochloride should not be less than 2000;
[0108] Determination method: 10 tablets of this product, grind finely, take the powder equivalent to 50mg of meclizine hydrochloride, accurately weigh it, put it in a 50ml volumetric flask, use the mixed solution of mobile phase A and B as the diluent, wherein A is 90% , dissolve with a diluent and dilute to the mark, shake well, filter, and take the subsequent filtrate as the test solution; accurately weigh the m...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap