High throughput cell-based HPV immunoassays for diagnosis and screening of HPV-associated cancers
一种细胞、癌症的技术,应用在用于诊断和筛选与HPV有关的癌症的高通量细胞基HPV免疫测定领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
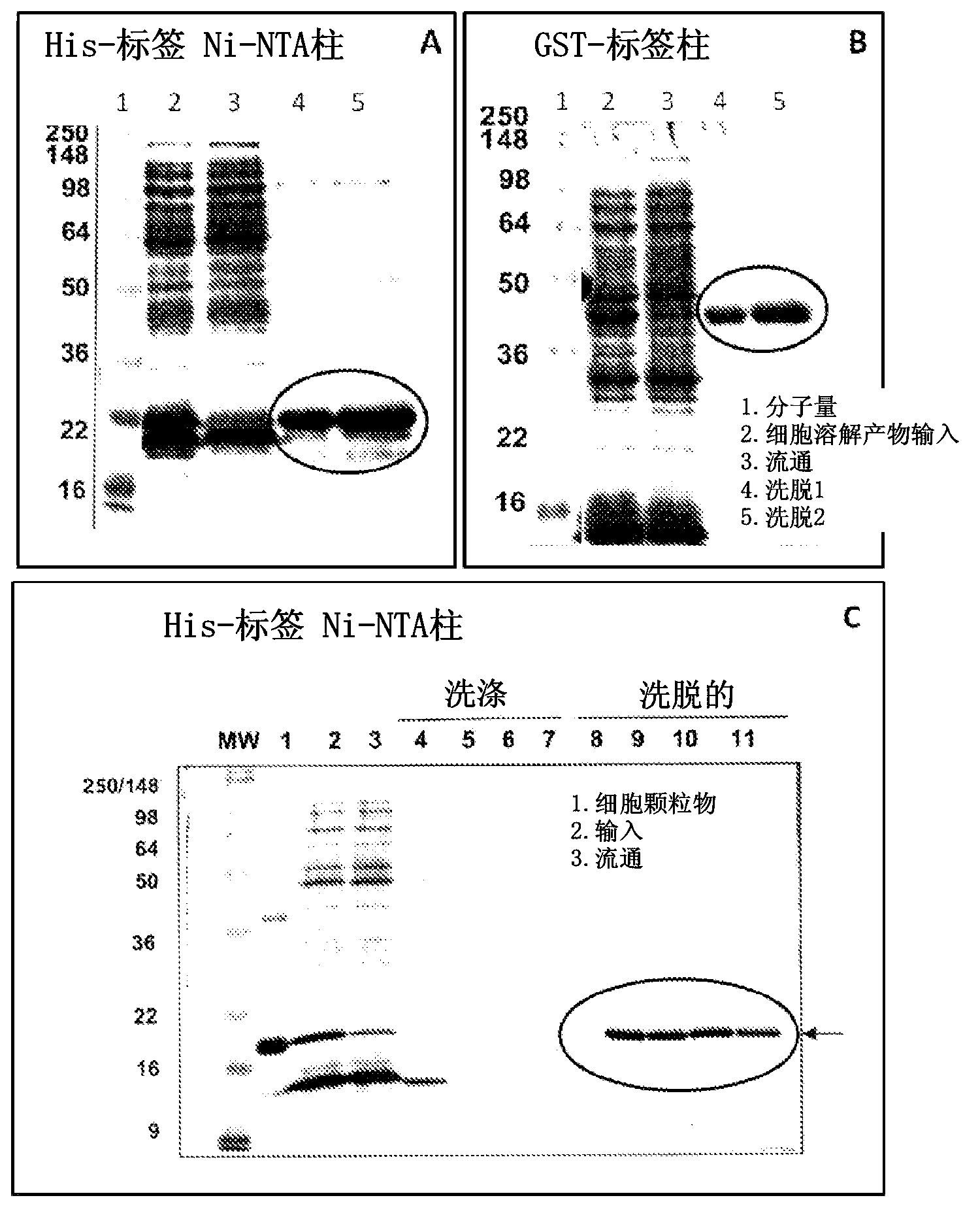
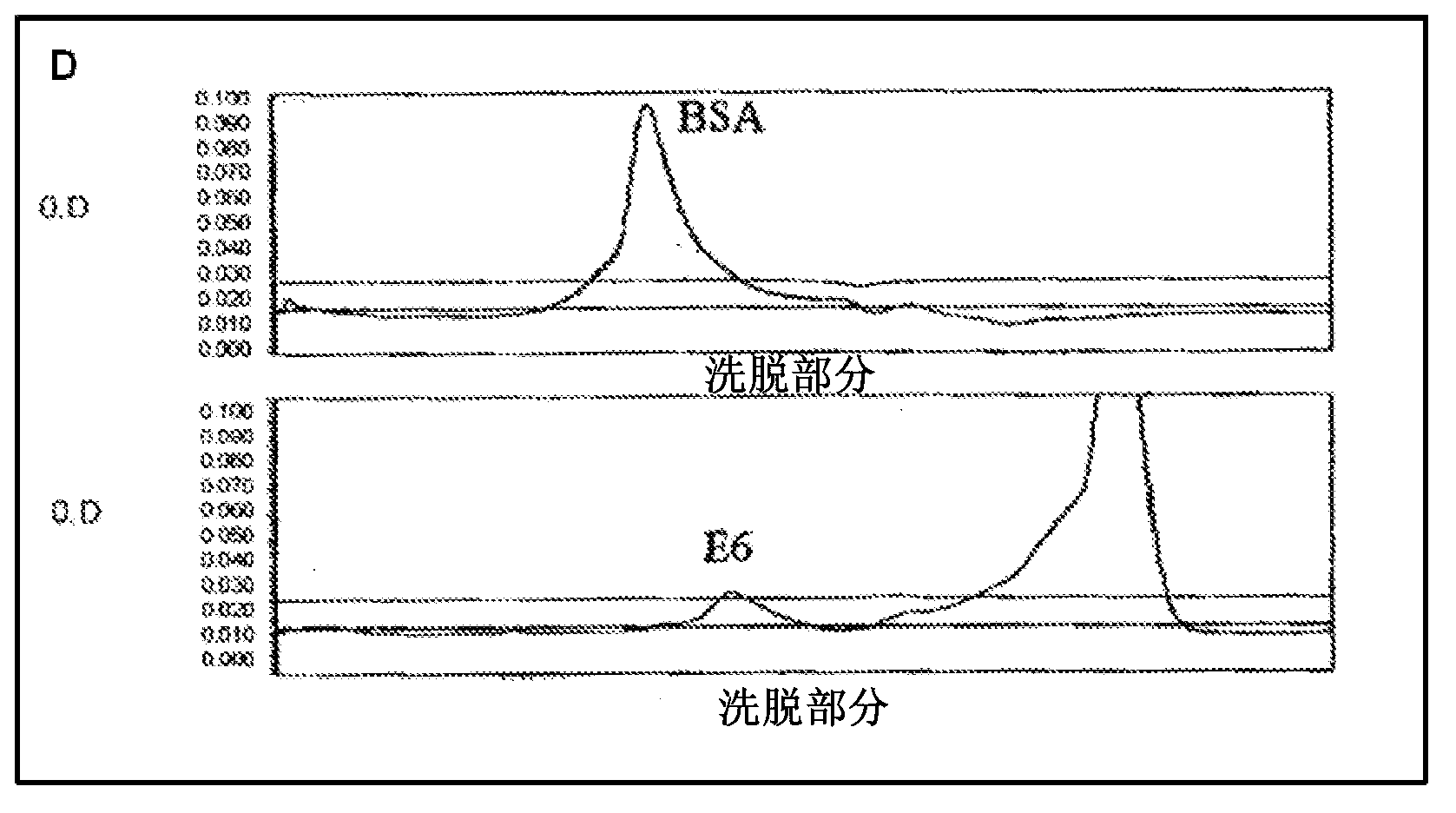
[0145] Example 1. Expression, purification and preparation of recombinant HPV proteins for use as immunogens to generate antisera and screen monoclonal antibodies from hybridoma cell lines
[0146] The HPV recombinant protein can be any kind of HPV protein, HPV protein of early gene and / or late gene, including but not limited to: E2, E6, E7, L1, L2, and can be from various HPV types. One aspect of the present invention provides a recombinant protein, such as a recombinant hybrid protein, which comprises a partial sequence or a full-length sequence of an HPV oncogene protein. For example, full length E6, E7 and / or L1 polypeptide sequences, which have been found difficult to obtain and purify due to undesired aggregation during protein purification, protein instability, low level expression, low immunogenic response of purified protein . For example, many early E6 oncoproteins contain many cysteine amino acids, thus correct topology of E6 oncoproteins requires proper formatio...
Embodiment 2
[0154] Example 2. Anti-HPV antibody preparation. Recombinant HPV proteins produced using the techniques described in Example 1 were used as immunogens to generate antisera, polyclonal antibodies, and monoclonal antibodies.
[0155] Basic techniques for performing cloning and performing immunoassays can be found in: "Antibodies: A Laboratory Manual", Harlow and Lane, Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y. 1989;" Molecular Cloning", A Laboratory Manual, eds. Sambrook, Fritsch and Maniatis, Cold Spring Harbor Laboratory Press, 1989 and other books and technical manuals known in the art. Details of our antibody production process to characterize the antibodies produced and certain assays are described in our co-owned U.S. Application Serial No. 12 / 456,053 - filed June 10, 2009, entitled "Novel Monoclonal Antibodies against HPV Proteins (for Novel Monoclonal Antibodies to HPV Proteins), U.S. Application Serial No. 12 / 456,054 - filed June 10, 2009, entitled "In si...
Embodiment 26
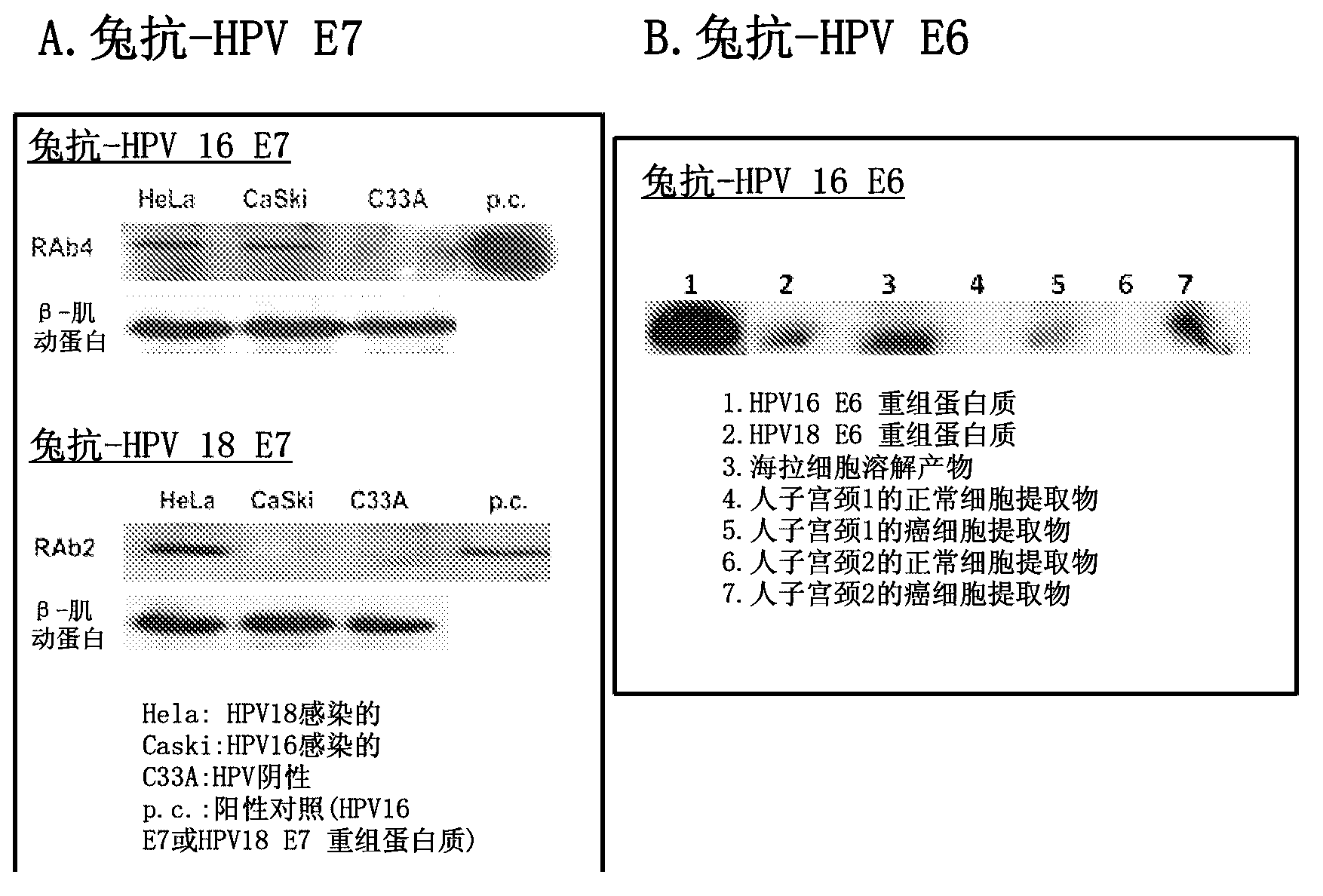
[0157] Example 2.6. Specificity of anti-HPV antibodies.
[0158]One or more immunoassays can be used to test the specificity of monoclonal antibodies produced by screening hybridoma cell lines with two or more HPV recombinant proteins. EIA (enzyme immunoassay) and / or Western blot were used as assay formats to test the specificity of the HPV antibodies described herein. Various purified recombinant HPV proteins—including the initial screening proteins used to obtain anti-HPV antibodies and other proteins not used in the screening—were used to coat microtiter plates to test the anti-HPV antibodies obtained by EIA. specificity. Proteins in cell lysates of cervical cancer cell lines (with or without HPV infection) were used to test the specificity of anti-HPV antibodies by Western blot. To determine the binding and reactivity of HPV antibodies to proteins from HPV-infected cell lines, Western blots are very helpful in showing specific protein bands corresponding to proteins pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com