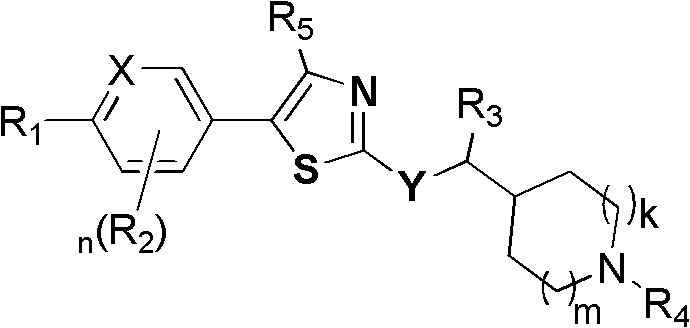
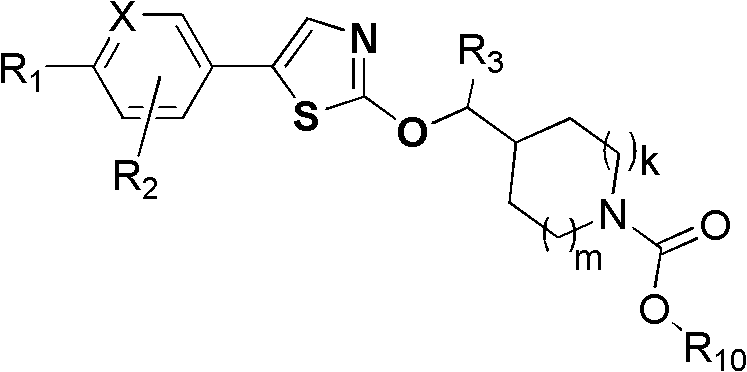
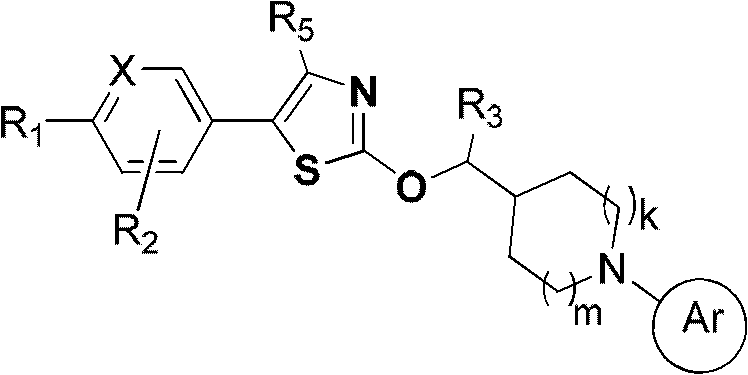
GPR119 agonist and application thereof
A solvate and selected technology, applied in the field of medicine, can solve the problems of decreased GIP activity, loss of sensitivity, and unclear exact reasons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] tert-butyl 4-((5-(4-methylsulfonylphenyl)thiazol-2-yloxy)methyl)piperidine-1-carboxylate
[0198]
[0199] Intermediate 1 (200 mg, 0.53 mmol), p-methylsulfonylphenylboronic acid (233 mg, 1.16 mmol), palladium acetate (35 mg, 0.15 mmol), triphenylphosphine (167 mg, 0.63 mol), sodium carbonate (416 mg, 3.92 mmol) were dispersed in 1,4-dioxane (4 ml) / water (1 ml), heated to 80°C under the protection of argon, and stirred for 2 hours , cooled to room temperature, filtered, and the filtrate was concentrated, followed by silica gel column chromatography to obtain a light yellow solid product (140 mg, 58%). 1 H NMR (CDCl 3 )δ (ppm): 7.93 (d, J=8.4Hz, 2H), 7.61 (d, J=8.4Hz, 2H), 7.46 (s, 1H), 4.31 (d, J=6.3Hz, 2H), 4.16 -4.23(m, 2H), 3.07(s, 3H), 2.74(t, J=12.3Hz, 2H), 2.00-2.07(m, 1H), 1.80(d, J=12.6Hz, 2H), 1.46( s, 9H), 1.27-1.35 (m, 2H). MS (m / z) 453 (M+1), 475 (M+23).
Embodiment 2
[0201] tert-butyl 4-((5-(3-fluoro-4-methanesulfonylphenyl)thiazol-2-yloxy)methyl)piperidine-1-carboxylate
[0202]
[0203] Intermediate 1 (80 mg, 0.21 mmol), Intermediate 7 (102 mg, 0.46 mmol), palladium acetate (14 mg, 0.06 mmol), triphenylphosphine (67 mg, 0.25 mmol), carbonic acid Sodium (166 mg, 1.56 mmol) was dispersed in 1,4-dioxane (4 mL) / water (1 mL), heated to 80 ° C under argon protection, stirred for 2 hours, and cooled to room temperature , filtered, and the filtrate was concentrated and then silica gel column chromatography to obtain a white solid product (50 mg, 50%). 1 H NMR (CDCl 3 )δ(ppm): 7.92(t, J=7.8Hz, 1H), 7.47(s, 1H), 7.35(d, J=8.1Hz, 1H), 7.28(d, J=7.8Hz, 1H), 4.32 (d, J=6.6Hz, 2H), 4.14-4.23(m, 2H), 3.23(s, 3H), 2.74(t, J=12.6Hz, 2H), 1.99-2.07(m, 1H), 1.79( d, J=12.3Hz, 2H), 1.46(s, 9H), 1.25-1.33(m, 2H). MS(m / z) 493(M+23).
Embodiment 3
[0205]tert-butyl 4-((5-(4-(tetrazol-1-yl)phenyl)thiazol-2-yloxy)methyl)piperidine-1-carboxylate
[0206]
[0207] Intermediate 1 (48 mg, 0.12 mmol), Intermediate 9 (69 mg, 0.25 mmol), palladium acetate (9 mg, 0.03 mmol), triphenylphosphine (40 mg, 0.15 mmol), carbonic acid Sodium (97 mg, 0.91 mmol) was dispersed in 1,4-dioxane (4 mL) / water (1 mL), heated to 80°C under argon protection, stirred for 1.5 hours, and cooled to room temperature , filtered, and the filtrate was concentrated, followed by silica gel column chromatography to obtain a white solid product (25 mg, 44%). 1 H NMR (CDCl 3 )δ (ppm): 9.00 (s, 1H), 7.72 (d, J = 8.1Hz, 2H), 7.63 (d, J = 8.1Hz, 2H), 7.41 (s, 1H), 4.32 (d, J = 6.6Hz, 2H), 4.14-4.23(m, 2H), 2.75(t, J=12.9Hz, 2H), 2.01-2.04(m, 1H), 1.81(d, J=12.6Hz, 2H), 1.47( s, 9H), 1.34-1.38 (m, 2H). MS (m / z) 443 (M+1), 465 (M+23).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com