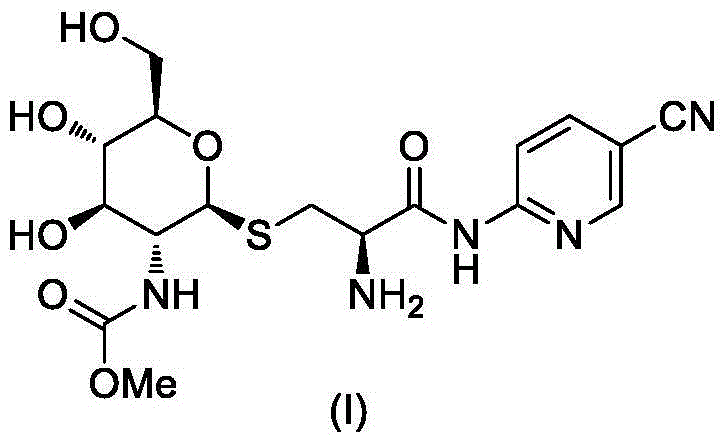
Derivative containing glucosamine and nitrile pyridine structure and use thereof
A technology for diabetes drugs and products, which is applied in the preparation of sugar derivatives, sugar derivatives, and sugar derivatives, etc., can solve the problems of unclear exact causes, loss of sensitivity, and decreased insulin secretion-stimulating effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 compound I-1
[0026]
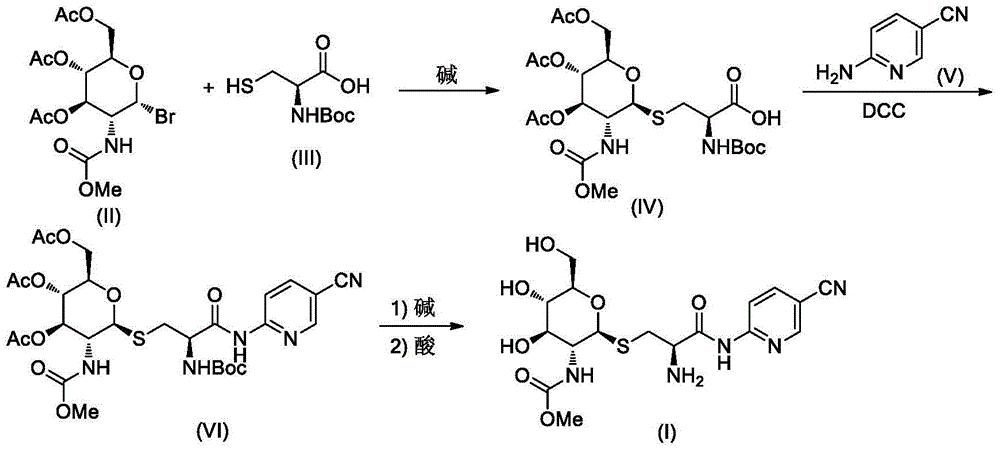
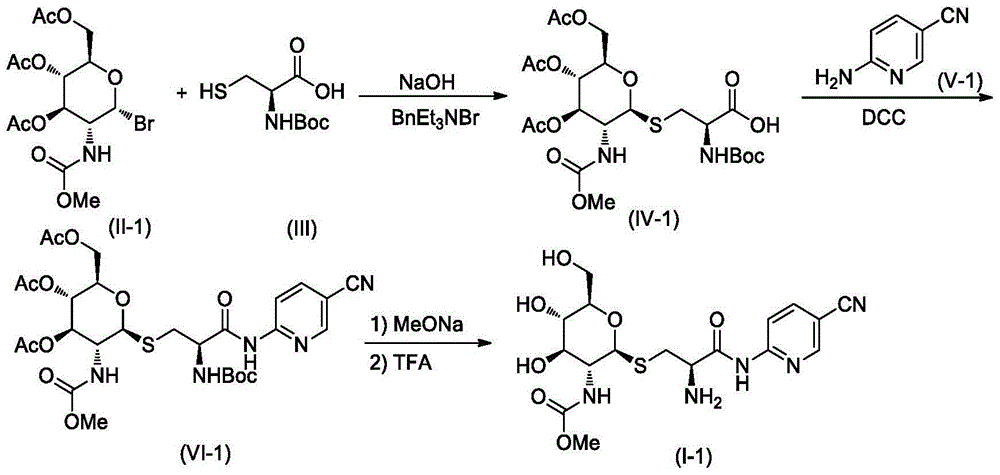
[0027] A. Synthesis of Compound IV-1
[0028] Compound II-14.26g (10mmol) and compound III 2.21g (10mmol) were dissolved in 20mL of dichloromethane, stirred at room temperature, 20mL of 20% NaOH solution and 3g of benzyltriethylammonium bromide were added, and then the reaction mixture was heated at room temperature After stirring overnight, the reaction was checked by TLC for completion. The reaction mixture was poured into 100 mL of ice water, carefully adjusted to pH=2-3 with hydrochloric acid, and 50 mL×3 of CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV-1 as a white solid, ESI-MS, m / z=567 ([M+H] + ).
[0029] B. Synthesis of Compound VI...
Embodiment 2
[0033] The preparation of embodiment 2 reference compound D-1
[0034] In order to further illustrate the pharmacological effects, the applicant listed a new compound D-1 (unpublished) and its pharmacological effects researched by the same applicant as a reference.
[0035]
[0036] A. Synthesis of Compound IV-2
[0037] Compound II-23.95g (10mmol) and compound III 2.21g (10mmol) were dissolved in 20mL of dichloromethane, stirred at room temperature, 20mL of 20% NaOH solution and 3g of benzyltriethylammonium bromide were added, and the reaction mixture was heated at room temperature After stirring overnight, the reaction was checked by TLC for completion. The reaction mixture was poured into 100 mL of ice water, carefully adjusted to pH=2-3 with hydrochloric acid, and 50 mL×3 of CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evap...
Embodiment 3
[0042] The agonistic experiment of the compound of Example 3 on GPR119 in vitro
[0043] Three copies of a human-mouse chimeric GPR119 expression construct encoding the FLAG epitope tag, the first 198 amino acids of human GPR119, and the C-terminal 137 amino acids of the mouse receptor were cloned into the tetracycline-inducible vector pcDNA5 / FRT / TO (Invitrogen #V6520-20), which contains a hygromycin resistance marker. Finely controlled expression of the receptor was achieved by stable integration of this construct into the genome of a specific host cell line Flp-In-T-Rex-HEK293 (Invitrogen) expressing a tetracycline repressor. Once a stable hygromycin-resistant cell line is generated, maintain the cells at 37 °C in humidified 5% CO 2 atmosphere and medium. The medium consisted of Dulbecco's modified Eagle's medium supplemented with 2 mM L-glutamine, 10% fetal calf serum, 200 μg / ml hygromycin B and 15 μg / ml blasticidin. medium) (DMEM, Invitrogen).
[0044] Cells stably ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com