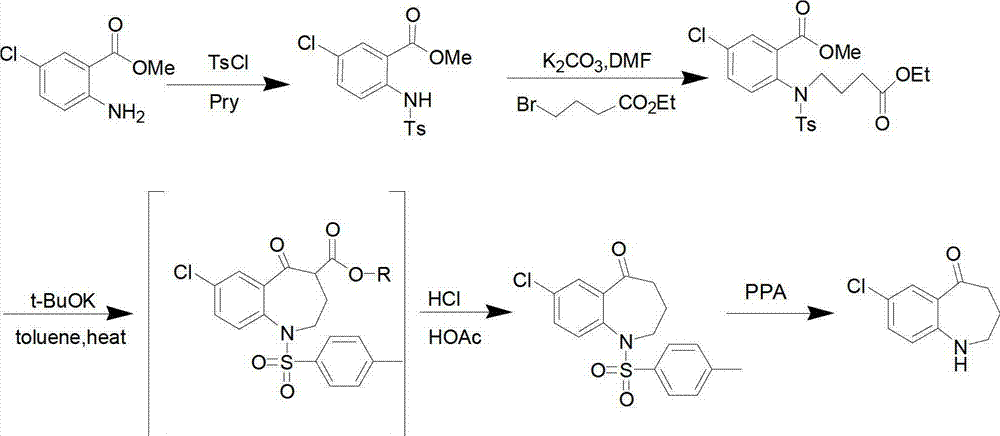
Preparation method of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine
A technology of benzonitrogen and oxo generation, applied in the field of chemical pharmacy, can solve the problems of easy moisture absorption, high viscosity of PPA, unsatisfactory yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014]
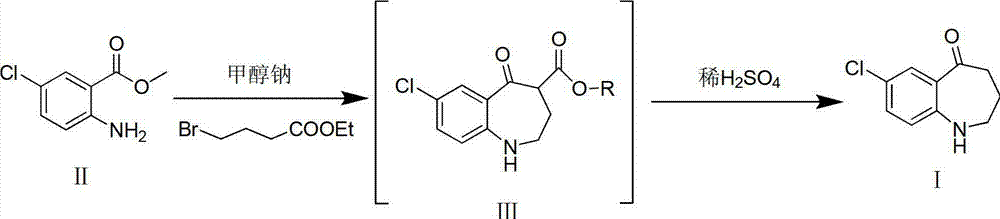
[0015] Add compound II (10g, 53.9mmol) and sodium methoxide (5.8g, 107.8mmol) into a 250ml reaction flask, add 100ml of DMF and stir to dissolve. mmol) in DMF (50ml) was slowly added dropwise to the reaction solution. After the dropwise addition, react at -20°C for 3h, then raise the temperature to 150°C for 1h. TLC [developing solvent: ethyl acetate-petroleum ether (1:5), the same below] After the detection reaction is complete, the reaction solution is poured into ice water, a white solid is precipitated, and the solid is filtered out. The obtained white solid was dissolved in dichloromethane, washed with saturated brine (30ml×3), the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain white solid III.
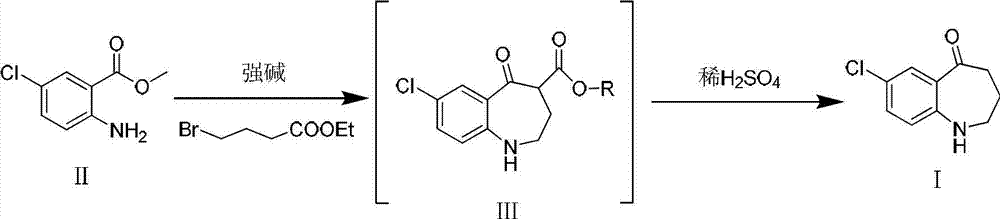
[0016] Put the obtained white solid III in a 250ml reaction bottle, add 100ml of 25% dilute sulfuric acid, react at 60°C for 2h, after TLC detection shows that the reaction ...
Embodiment 2
[0018]
[0019] Add compound II (10g, 53.9mmol) and sodium ethoxide (5.5g, 80.8mmol) into a 250ml reaction flask, add 100ml of acetonitrile and stir to dissolve, at -10°C, add 4-bromobutyric acid ethyl ester (10.5g, 53.9 mmol) of acetonitrile solution (50 ml) was slowly added dropwise to the reaction solution. After the dropwise addition, react at 20°C for 0.5h, then raise the temperature to 80°C for 6h. After the completion of the reaction as detected by TLC, the reaction solution was poured into ice water, a white solid was precipitated, and the solid was filtered out. The obtained white solid was dissolved in dichloromethane, washed with saturated brine (30ml×3), the organic layers were combined, dried over anhydrous sodium sulfate, filtered, and the solvent was evaporated under reduced pressure to obtain white solid III.
[0020] Put the obtained white solid III in a 250ml reaction bottle, add 100ml of 15% dilute sulfuric acid, and react at 80°C for 3h. 2 CO 3 soluti...
Embodiment 3
[0022]
[0023] Add compound II (10g, 53.9mmol) and potassium tert-butoxide (6.1g, 53.9mmol) into a 250ml reaction flask, add 100ml of toluene and stir to dissolve. At 0°C, ethyl 4-bromobutyrate (10.5g , 53.9 mmol) in toluene (50 ml) was slowly added dropwise to the reaction solution. After the dropwise addition, react at 0° C. for 6 h, then raise the temperature to reflux for 2 h. After the completion of the reaction as detected by TLC, the reaction solution was poured into ice water, extracted with dichloromethane (20ml×3), and the solvent was evaporated under reduced pressure to obtain white solid III.
[0024] Put the obtained white solid III in a 250ml reaction bottle, add 100ml of 20% dilute sulfuric acid, and react at 50°C for 2.5h. After the TLC test shows that the reaction is complete, cool to room temperature and adjust with KOH solution (1mol / L) in an ice bath. The pH of the reaction system was 7~8, and vacuum filtration gave the target product 7-chloro-5-oxo-2,...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap