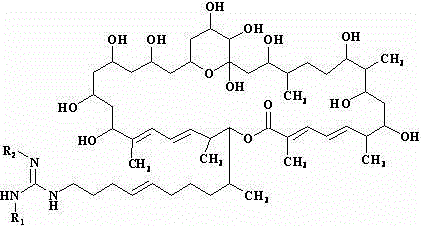
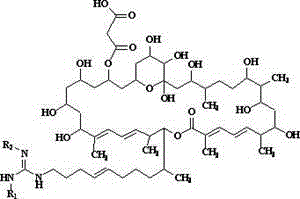
De-malonate monoacyl azalomycin F, preparation method thereof and application thereof to preparation of MRSA infection therapeutic drug
A technology for removing malonyl azamycin and malonyl azamycin, which is applied in the application field of preparing medicines for treating MRSA infection, and can solve the problems of loss of antimicrobial activity, poor solubility in water, and structural inadequacy. Stability and other issues, to achieve the effects of enhanced stability, significant inhibition and killing, and increased water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 100 mg of sodium hydride in 100 ml of methanol, and add 1.1 g (1 mmol) of azamycin F under stirring 5a , Stir bar reaction at 0°C for 12 h. The reaction mixture was neutralized with 5 M hydrochloric acid aqueous solution, concentrated under reduced pressure at 40°C, and the concentrate was separated by C-18 reverse-phase high-performance liquid chromatography, eluted with methanol-water solution with a volume ratio of 80:20, and the collected rich Malonyl azamycin F 5a The eluate was concentrated under reduced pressure at 37°C and freeze-dried to obtain 0.83 g demalonyl azamycin F 5a (1).
[0024] demalonyl azamycin F 5a (1): Molecular formula is C 54 h 95 N 3 o 14 , soluble in water, easily soluble in methanol and ethanol, m.p. 126.0~128.0℃, ESI-MS ( m / z ): 1010.7 [M+H] + . 13 C NMR (MeOH- d 4 , 100 MHz) δ ppm: 169.2(s), 157.4(s), 145.0(d), 139.3(s), 137.0(s), 135.3(d), 132.0(d), 129.4(d), 128.6(d), 127.4(d ), 127.0(d), 126.3(d), 99.7(s), 80.3(...
Embodiment 2
[0026] Dissolve 100 mg of sodium hydride in 100 ml of methanol, and add 1.1 g (1 mmol) of azamycin F under stirring 4a , Stir bar reaction at 5 °C for 8 h. The reaction mixture was neutralized with 5 M hydrochloric acid aqueous solution, concentrated under reduced pressure at 40°C, and the concentrate was separated by C-18 reverse-phase high-performance liquid chromatography, eluted with methanol-water solution with a volume ratio of 78:22, and the rich Malonyl azamycin F 4a The eluate was concentrated under reduced pressure at 37°C and freeze-dried to obtain 0.79 g demalonyl azamycin F 4a (2).
[0027] demalonyl azamycin F 4a (2): Molecular formula is C 53 h 93 N 3 o 14, soluble in water, easily soluble in methanol and ethanol, m.p. 126.5~127.7℃, ESI-MS ( m / z ): 996.7 [M+H] + . 13 C NMR (MeOH- d 4 , 100 MHz) δ ppm: 169.1(s), 158.3(s), 145.1(d), 139.3(s), 137.0(s), 135.2(d), 132.0(d), 129.4(d), 128.5(d), 127.4(d) ), 127.0(d), 126.3(d), 99.8(s), 80.3(d), 77.1(d)...
Embodiment 3
[0029] Dissolve 200 mg of sodium hydride in 250 ml of methanol, and add 2.1 g (2 mmol) of azamycin F under stirring 3a , Stir bar reaction at -5°C for 18 h. The reaction mixture was neutralized with 5 M hydrochloric acid aqueous solution, concentrated under reduced pressure at 40°C, and the concentrate was separated by C-18 reverse-phase high-performance liquid chromatography, eluted with methanol-water solution with a volume ratio of 78:22, and the rich Malonyl azamycin F 3a The eluate was concentrated under reduced pressure at 37°C and freeze-dried to obtain 1.5 g demalonyl azamycin F 3a (3).
[0030] demalonyl azamycin F 3a (3): Molecular formula is C 52 h 91 N 3 o 14 , soluble in water, easily soluble in methanol and ethanol, m.p. 126.3~127.5℃, ESI-MS ( m / z ): 982.6 [M+H] + . 13 C NMR (MeOH- d 4 , 100 MHz) δ ppm: 169.1(s), 158.7(s), 145.1(d), 139.3(s), 137.0(s), 135.2(d), 132.0(d), 129.4(d), 128.5(d), 127.4(d) ), 127.0(d), 126.3(d), 99.9(s), 80.3(d), 77.2(d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com