Soft capsule for treating nerve diseases
A technology of soft capsules and soft capsule shells, applied in nervous system diseases, capsule delivery, blood diseases, etc., can solve problems such as left ventricular enlargement and reduction, improve subjective symptoms, improve brain energy metabolism, and inhibit the production of lipid peroxides qualitative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1: prepare soft capsule of the present invention
[0060] Liquid formula:
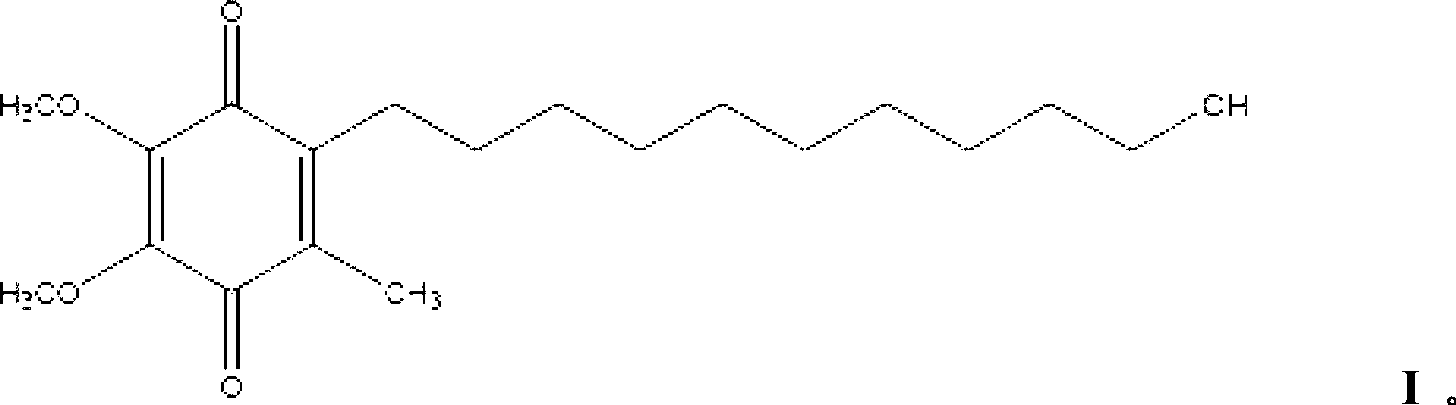
[0061] Idebenone:
6g
Soybean oil: appropriate amount, add to
100g
[0062] Softgel Shell Formulation:
[0063] Gelatin:
100g
Glycerin:
50g
water:
100g
[0064] Preparation steps:
[0065] (1) Preparation of medicinal solution: prepare idebenone and auxiliary materials into medicinal solution at room temperature;
[0066] (2) Preparation of soft capsule shell material: mixing and dissolving gelatin, plasticizer, and water to make a soft capsule shell material liquid;
[0067](3) Preparation of soft capsules: use the dropping method, the content weight of each capsule is 500mg (those skilled in the art can easily adjust and control the content within the scope of 200mg~2000mg / granule, such as controlling to about 250mg, about 500mg, about 750mg, about 1000mg, about 1500mg, etc. Or control the amount of activ...
Embodiment 2
[0069] Embodiment 2: prepare soft capsule of the present invention
[0070] Liquid formula:
[0071] Idebenone:
[0072] Softgel Shell Formulation:
[0073] Gelatin:
[0074] Preparation steps: carry out with reference to the method of Example 1.
Embodiment 3
[0075] Embodiment 3: prepare soft capsule of the present invention
[0076] Liquid formula:
[0077] Idebenone:
[0078] Softgel Shell Formulation:
[0079] Gelatin:
[0080] Preparation steps: carry out with reference to the method of Example 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap