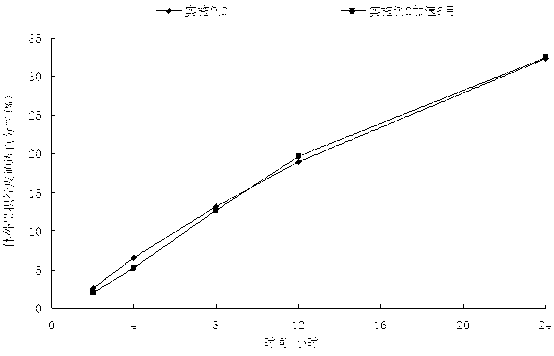Oxybutynin transdermal gel and preparation method thereof
A gel and transdermal technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as adverse effects on the skin, and achieve improved clinical treatment effects, no foreign body sensation, and good air permeability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
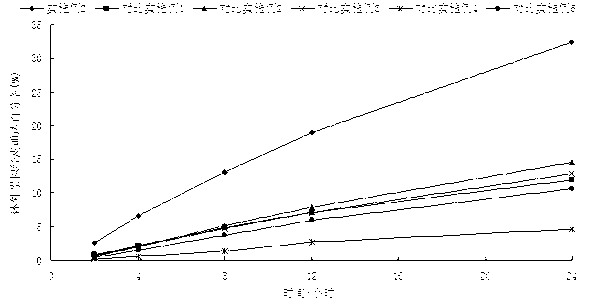
Image
Examples
Embodiment 1
[0040] The present embodiment is the preparation of the oxybutynin hydrochloride gel that main ingredient content is 1%:
[0041] prescription composition Prescription amount Oxybutynin Hydrochloride 1g Absolute ethanol 70g HPMC E4M 1.5g glycerin 2g sodium hydroxide Appropriate amount purified water Add to 100g
[0042] Preparation method: HPMC E4M is passed through a 80-mesh sieve, oxybutynin hydrochloride is dissolved in 30% prescription amount of absolute ethanol to form an ethanol solution of oxybutynin hydrochloride, and NaOH is prepared with purified water to make a 5mol / L NaOH solution for later use; Appropriate amount of purified water, add HPMC E4M into water to make it swell into a uniform colloid, add glycerin, 70% prescription amount of absolute ethanol and oxybutynin hydrochloride ethanol solution respectively under stirring, after mixing evenly, add NaOH solution to adjust When the pH value reaches 5.6, add th...
Embodiment 2
[0044] The present embodiment is the preparation of the oxybutynin hydrochloride gel that main ingredient content is 10%:
[0045] prescription composition Prescription amount Oxybutynin Hydrochloride 10g Absolute ethanol 70g HPMC E4M 1.5g glycerin 2g sodium hydroxide Appropriate amount purified water Add to 100g
[0046] Preparation method: HPMC E4M is passed through a 80-mesh sieve, oxybutynin hydrochloride is dissolved in 30% prescription amount of absolute ethanol to form an ethanol solution of oxybutynin hydrochloride, and NaOH is prepared with purified water to make a 5mol / L NaOH solution for later use; Appropriate amount of purified water, add HPMC E4M into water to make it swell into a uniform colloid, add glycerin, 70% prescription amount of absolute ethanol and oxybutynin hydrochloride ethanol solution respectively under stirring, after mixing evenly, add NaOH solution to adjust When the pH value reaches 5.6, add ...
Embodiment 3
[0048] The present embodiment is the preparation of the oxybutynin hydrochloride gel that main ingredient content is 30%:
[0049] prescription composition Prescription amount Oxybutynin Hydrochloride 30g Absolute ethanol 50g HPMC E4M 1.5g glycerin 2g sodium hydroxide Appropriate amount purified water Add to 100g
[0050] Preparation:
[0051] HPMC E4M passes through a 80-mesh sieve, dissolves oxybutynin hydrochloride in 30% prescription amount of absolute ethanol to form an ethanol solution of oxybutynin hydrochloride, and NaOH is prepared with purified water to make a 5mol / L NaOH solution for later use; take an appropriate amount of purified water , add HPMC E4M into water to make it swell into a uniform colloid, add glycerin, 70% prescription amount of absolute ethanol and oxybutynin hydrochloride ethanol solution respectively under stirring, after mixing evenly, add NaOH solution to adjust the pH value to 5.6, add the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com